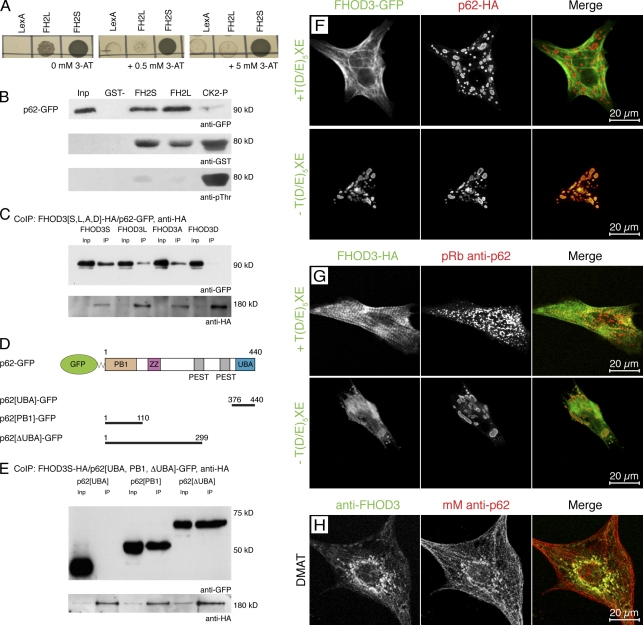
Figure 3.
FHOD3 interacts with the p62-PB1 domain in a phosphorylation-regulated manner. (A) Yeast two-hybrid assay to test for the specificity and strength of the p62 interaction with the FHOD3-FH2 domain. p62-pGAD10 was transformed into yeast together with FH2L and FH2S-LexA, as well as with the empty LexA vector as a control, and grown on medium containing 0, 0.5, and 5.0 mM of 3-AT. p62 strongly interacts with FH2S and interacts much more weakly with FH2L. (B) GST pull-down assays to analyze the FHOD3–p62 interaction. Equal amounts of purified FH2L-GST, FH2S-GST, or CK2-phosphorylated FH2L-GST (CK2-P) were incubated with lysates of p62-GFP–transfected COS-1 cells and subjected to immunoblotting with monoclonal mouse (mM) anti-GFP and, subsequently, polyclonal goat anti-GST. The successful phosphorylation of FH2L-GST was controlled by incubation with monoclonal mouse anti–phospho-threonine (pThr). Although the presence of the unphosphorylated T(D/E)5XE exon has no effect on the interaction with p62, CK2 phosphorylation of FH2L-GST before the incubation strongly reduces the interaction. Inp, input. (C) Coimmunoprecipitation (IP; CoIP) of full-length FHOD3 and p62. Lysates of COS-1 cells, which were transfected with p62-GFP and FHOD3-HA constructs containing (FHOD3L) or excluding (FHOD3S) the T(D/E)5XE exon, as well as with constructs with residues T1474 and T1476 mutated to alanine (FHOD3A) or aspartic acid (FHOD3D), were immunoprecipitated with anti-HA and subjected to immunoblotting with anti-GFP and subsequently anti-HA antibodies. Mutation of the threonine residues to aspartic acid, but not to alanine, greatly reduced the interaction. (D) p62 domain structure and transfection constructs. Domains were constructed according to Simple Modular Architecture Research Tool (SMART) and Geetha and Wooten (2002). ZZ, zinc finger domain (ZZ type). (E) To identify the minimal binding site, COS-1 cells were cotransfected with FHOD3S-HA and p62-GFP constructs containing the PB1 domain, the UBA domain, or the protein without the UBA domain (ΔUBA) as indicated in D. Lysates were treated as described earlier. Binding was found with the ΔUBA and the PB1 domain, indicating that binding of FHOD3 requires presence of the PB1 domain in p62. (F) Cotransfection of NRCs with p62-HA and FHOD3-GFP constructs. Cells were stained with monoclonal rat anti-HA. p62 recruits FHOD3 lacking the T(D/E)5XE exon into aggregates. (G) Transfection of NRCs with FHOD3-GFP constructs and staining with pRb anti-p62 show colocalization of endogenous p62 only with FHOD3 lacking the T(D/E)5XE exon. (H) Visualization of endogenous FHOD3 and p62 by immunofluorescence in NRCs that were treated with DMAT to inhibit CK2 demonstrates colocalization after the redistribution of FHOD3.