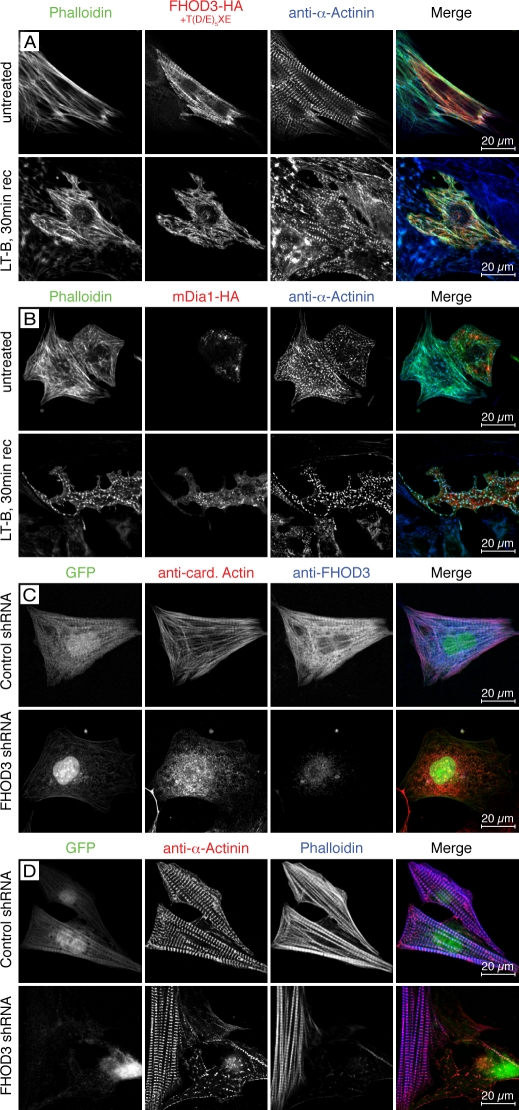
Figure 5.
FHOD3 is required for the polymerization of actin filaments in cardiomyocytes. (A–D) NRCs transfected with FHOD3-HA (including the T(D/E)5XE exon; A), mDia1-HA (B), or FHOD3-shRNA constructs (C and D) were treated with 20 µM latrunculin B (LT-B) overnight. Untreated transfected NRCs are shown in A and B (top), whereas control shRNA-transfected NRCs are shown in C and D (top). Latrunculin was removed, and cells were left to recover for 30 min (30 min rec; A and B) or 2 h (C and D). Cells were stained with monoclonal rat anti-HA, monoclonal mouse anti–α-actinin, and phalloidin (A and B) and monoclonal mouse anticardiac actin (anti-card. Actin) and pRb anti-FHOD3 (C) or monoclonal mouse anti–α-actinin and phalloidin (D). (A and B) Only cells transfected with FHOD3 have repolymerized their actin filaments after 30 min. Transfection with mDia1 results in a speckled phalloidin staining, indicating only reduced actin polymerization, whereas untransfected cells display no recovery of actin polymerization. (C) After 2 h, untransfected cells are starting to recover, with the majority of cardiac actin assembled into filaments. Colocalization with cardiac actin indicates an involvement of (endogenous) FHOD3 in the polymerization of F-actin. In contrast, no actin polymerization was detected after knockdown of FHOD3, further stressing the importance of this formin for the assembly of cardiac actin filaments (C and D).