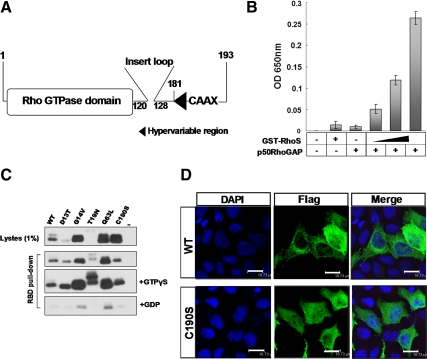
Figure 2.
The conservation of RhoS in the structure and enzymatic activity as a Rho GTPase. (A) A schematic structural model of RhoS. Numbers indicate corresponding amino acids in sequence. (B) GTP hydrolysis activity assay. Enzymatic activity was measured by GTP hydrolysis using purified GTPase protein. The phosphate generated by hydrolysis of GTP was measured by the addition of CytoPhos (Cytoskeleton) reagent and reading the absorbance at 650 nm. Results are presented as the mean ± SD from three experiments. (C) Activated Rho GTPase (GTP-bound) protein pulldown assay. Extracts were prepared from HEK293T cells transiently expressing Flag-tagged wild-type or mutated RhoS including RhoS(D13T), RhoS(G14V), RhoS(T19N), RhoS(Q63L), and RhoS(C190S). After a 1-h incubation with GST-RBD glutathione beads, the bound proteins were analyzed by Western blotting using anti-Flag antibody. (D) A conserved C-terminal site (C190) is essential for the proper localization of RhoS. Subcellular distributions of wild-type and C190S mutant were analyzed by immunofluorescence. Twenty-four hours after transfection, MCF-7 cells transfected with the indicated plasmids were prepared for immunofluorescence analysis using anti-Flag antibody. Bar, 18.73 μm.