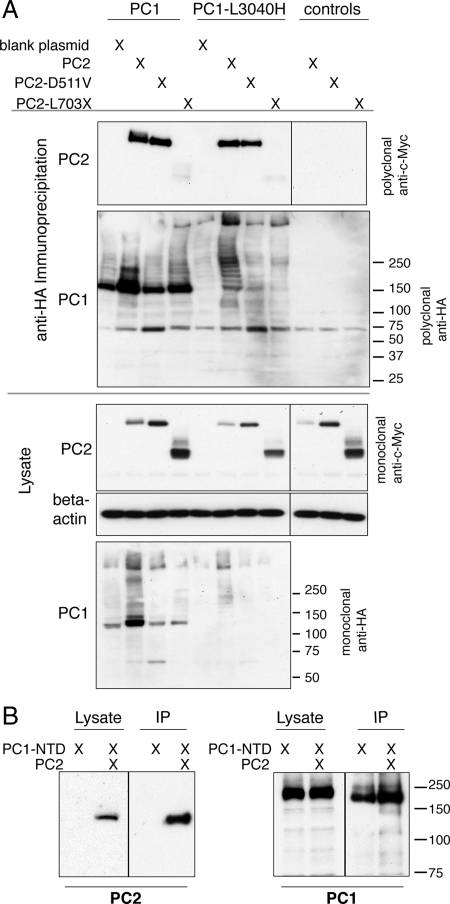
Figure 5.
Both PC1 and PC1-L3040H stably bind PC2 and PC2-D511V, whereas PC2-L703X fails to coimmunoprecipitate with either PC1 construct. Protein complexes containing PC1 or L3040H were immunoprecipitated (IP'd) using an antibody against the C-terminal HA tag on PC1 and then the immunoprecipitates were Western blotted with polyclonal anti-c-Myc antibody to detect the presence of the c-Myc epitope appended to PC2 (A, top). Control conditions in which PC2 constructs were expressed in the absence of PC1 revealed that there is no nonspecific signal from the anti-c-Myc antibody (A, right-most lanes). PC2 and PC2-D511V stably interacted with both PC1 constructs, whereas PC2-L703X failed to be pulled down with PC1 or PC1-L3040H (A, top). This failure of PC2-L703X to coIP was not due to a lack of PC1 construct pull-down, as shown by blotting the IP with polyclonal anti-HA antibody (A). PC1-NTD also robustly bound to PC2, as seen by PC2's presence in the pull-down from an IP with the anti-HA antibody against PC1-NTD (B, left). The success of the IP is revealed by Western blotting with anti-HA antibody, which detects PC1-NTD in both the lysate and IP material (B, right).