Figure 3.
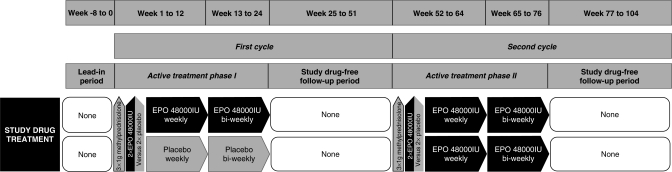
Investigational drug treatment design of ‘recombinant human erythropoietin as a neuroprotective/-regenerative treatment of chronic progressive multiple sclerosis (EPO-MS): a double-blind, placebo-controlled, randomized phase IIb trial’. Patients start with an 8-week lead-in period, allowing extensive, repeated rating of baseline performance (estimation of fluctuations) ended by a short, dense ‘start-up assessment’ (including clinical, neurophysiological, neuroophthalmological, MRI, cognitive, laboratory assessments). Treatment initiation with 3 × 1000 mg methylprednisolone given on 3 consecutive days, and EPO (48,000 IU; in additional dose-finding treatment arms alternative dosing with 32,000 and 16,000 IU, respectively) vs placebo on days 2 and 3 of methylprednisolone treatment follows. Patients then receive EPO/placebo weekly for 12 weeks, biweekly for another 12 weeks and undergo a 6-month post-treatment observation period with visits every 6 weeks. In the second year all patients are switched to verum following the same schedule until individual study end after 26 months (2 + 12 + 12).