Abstract
The treatment of hepatocellular carcinoma (HCC) remains a challenge, with 1- and 3-year survival rates of 20% and 5%, respectively, and a median survival of 8 months. However, a better understanding of the pathogenesis of HCC, and advances in targeted molecular therapies provide physicians treating this disease with new hope. The treatment of HCC is multidisciplinary, requiring surgeons, hepatologists, interventional radiologists and oncologists. Thus, there is enormous potential to combine various treatment modalities to improve survival for patients. This review will describe what is currently known about the molecular pathogenesis of HCC, explore current and future treatments based on these pathways, and describe how these new therapies fit into existing approaches to HCC treatment.
Keywords: hepatocellular carcinoma (HCC), treatment, molecular therapies, novel agents
Introduction
Recent clarification of several molecular pathways associated with the development of hepatocellular carcinoma (HCC) has coincided with the identification of new targeted therapies. These treatments may be used as monotherapy for tumors not amenable to surgical resection or transplant. They are also being studied as adjunctive therapies combined with other modalities. This article will review several of the pathways identified in the pathogenesis of HCC, highlighting specific targeted therapies and describing how these new agents may alter our current treatment approach for HCC.
Epidemiology
HCC is the fifth most common cancer worldwide and the third most common cause of cancer-related deaths globally [Bosch et al. 2005; Pisani et al. 2002]. The incidence of HCC in the United States has almost doubled in recent decades [El-Serag et al. 1999]. HCC usually occurs in the setting of underlying liver diseases, including hepatitis B and C, nonalcoholic steatohepatitis (NASH), hemochromatosis, autoimmune hepatitis, alcohol-related liver disease, primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC). Thus, the patient population is diverse, contributing to the complexity of studying this tumor. The majority of patients who develop HCC in the West have underlying cirrhosis, due primarily to HCV, alcohol, and increasingly, to NASH.
Screening
The American Association for the Study of Liver Diseases (AASLD) guidelines suggest that patients with cirrhosis be screened for HCC with biannual imaging (CT, MRI or ultrasound) and alfa-fetoprotein (AFP) levels [Bruix and Sherman, 2005]. For patients with Hepatitis B Virus (HBV), it is not necessary to have cirrhosis prior to the development of HCC, so screening recommendations are unique for these patients.
Noncirrhotic patients with hepatitis B surface antigen positivity (HBsAg +), Asian males >40 years of age, Asian females >50 years of age, and Africans >20 years should all be screened, together with those with hepatitis B and a family history of HCC. Additionally, patients with a very high viral load may be at increased risk for developing HCC, but there are no specific screening guidelines for these patients [El-Serag et al. 2008]. These recommendations are based on the results of one randomized controlled trial of over 18,000 patients with HBV in China who were randomized to either surveillance with AFP and ultrasound every 6 months, or no surveillance. While compliance was poor in the study, 6-month surveillance was associated with a 37% reduction in HCC-related mortality [Zhang et al. 2004]. Although this finding has not been reproduced in subsequent studies, given the noninvasive nature of screening with AFP and ultrasound, this has become standard of care.
Diagnosis
The diagnosis of HCC incorporates AFP levels, imaging, and histology within the context of underlying liver disease. There remains some debate as to whether triple phase CT scan or MRI is more sensitive for the detection of HCC, and there is a significant amount of institutional variation, but it is generally thought that MRI is more sensitive than CT [De Ledinghen et al. 2002; Libbrecht et al. 2002; Rode et al. 2001]. Typical imaging findings of HCC include initial arterial enhancement with subsequent rapid venous washout. AASLD guidelines recommend that a diagnosis of HCC be made by imaging for lesions >2 cm with typical imaging characteristics in a patient with cirrhosis. A biopsy is required for lesions without these typical imaging qualities or for those detected in a noncirrhotic liver. Lesions between 1 and 2 cm may be diagnosed with two dynamic imaging studies, and those that are <1 cm are often followed closely, in conjunction with AFP levels until they either demonstrate clearer imaging characteristics, or are large enough to be biopsied [Bruix et al. 2005]. Although AFP is not sufficiently sensitive to be used as a screening tool for HCC alone, AFP levels >200 ng/ml in conjunction with characteristic imaging findings are specific enough to make a diagnosis of HCC in the absence of tissue [Bruix et al. 2005].
Novel biomarkers are also being looked at as a means of detecting HCC. In a recent case control study looking at AFP, des-γ carboxyprothrombin (DCP) and lectin-bound AFP (AFP-L3) in patients with HCC and those with cirrhosis, AFP was found to the most accurate marker for HCC [as assessed by area under the receiver operating characteristic (ROC) curve] in patients with early HCC. However, the cutoff of AFP used in this study was 10.9 ng/ml as this was the cutoff that maximized sensitivity and specificity on the ROC curve. DCP was also shown to be more predictive of HCC in later stages, but this is not optimal for a screening test [Marrero et al. 2009].
Transcriptional profiles are also being studied as an alternative diagnostic test for HCC. In one study, the trio of glypican-3 (a heparin sulfate proteoglycan shown to be upregulated in HCC), LYVE1 (a hyaluronan receptor expressed by endothelial cells), and survivin (an inhibitor of apoptosis) was shown to have a sensitivity of 95%, specificity of 94%, positive predictive value of 95% and negative predictive value of 94% in detecting HCC from dysplastic nodules [Llovet et al. 2006].
Models of pathogenesis
As discussed, patients who develop HCC represent a diverse group with a variety of underlying risk factors, including hepatitis B and C, alcohol, metabolic syndrome, and exposure to environmental toxins such as aflatoxin. Because of this, there is likely no one genetic mutation or molecular pathway that functions as a crucial step in all HCC tumorgenesis. Building on work done by Hanahan and Weinberg, Llovet and Bruix outline several mechanisms that are likely to be disrupted in HCC [Llovet and Bruix, 2008; Hanahan and Weinberg, 2000]. Examples include: (1) altered cell cycle regulation; (2) aberrant angiogenesis; (3) evasion of apoptosis; and (4) loss of intrinsic mechanisms to limit cell replication. This framework provides a context for studying specific genetic mutations, including loss of function mutations, altered methylation patterns, increased or decreased receptor activation, or telomere shortening, that may play a role in the development of HCC.
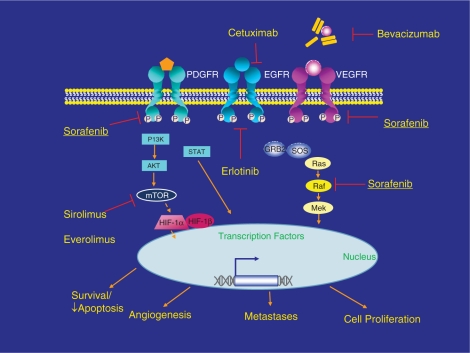
In addition to these mechanisms, several discrete signaling pathways have been identified in the development and progression of HCC. The majority of these pathways involve the activation of protein kinases, and it is these proteins and their receptors that have become the focus of intense efforts at developing molecular targeted therapies for HCC. We will describe some of these pathways as well as their potential therapeutic targets. Figure 1 is a simplified diagram of some of these pathways.
Figure 1.
Molecularly targeted therapy in hepatocellular carcinoma (HCC). Reprinted with permission of John Wiley & Sons, Inc. [Zhu, 2008].
VEGFR/EGFR-Ras pathway
HCC is one of the most vascular solid tumors known, and vascular endothelial growth factor (VEGF) seems to be a primary mediator of angiogenesis in HCC [Moon et al. 2003; Yoshiji et al. 1998]. Anti-antiangiogenic drugs such as bevacizumab (an anti-VEGF antibody), and sorafenib (which acts in part to block the VEGF tyrosine kinase receptor) have already shown significant clinical activity in HCC, and sorafenib is now the first FDA-approved treatment for patients with advanced HCC [Llovet et al. 2008a; Siegel et al. 2008a].
The epidermal growth factor receptor (EGFR) is also expressed by HCC in vitro and in vivo, and activation of the receptor is also involved in HCC carcinogenesis [Schiffer et al. 2005]. VEGF and the EGFR both activate the Ras pathway. Activation of Ras results in the transcription of several genes in the AP-1 family such as c-fos and c-jun. AP-1 is a transcription factor complex thought to be critical in the malignant transformation of cells as well as activation other oncogenes, particularly those in the Ras pathway [Ozanne et al. 2007].
Sorafenib
Sorafenib is a monoclonal antibody directed against several targets, including VEGFR and Ras. It has now been studied in two large multicenter, randomized, phase III placebo controlled trials in patients with advanced HCC. In Llovet’s Phase III Sorafenib HCC Assessment Randomized Protocol (SHARP) study, 602 patients were randomized to sorafenib 400 mg twice daily or placebo. The sorafenib group had a median overall survival time of 10.7 months compared with 7.9 months in the placebo group (p ≤ 0.001) [Llovet et al. 2008a]. In a second, randomized, placebo control study, 271 patients were randomized among 23 centers in Asia to sorafenib or placebo. Median overall survival in the sorafenib group was 6.5 months compared with 4.2 months for the placebo group (hazard ratio for treatment = 0.68, p = 0.014) [Cheng et al. 2009]. It is thought that the overall worsened survival in this trial may be due to more advanced disease on presentation, but interestingly, the degree of benefit for sorafenib in each study was almost identical. Results of these studies resulted in the FDA’s approval of sorafenib for treatment of advanced HCC.
Abou Alfa and colleagues looked at the use of sorafenib in patients including some with more advanced liver disease. In a phase II study of 137 patients, 39 of whom were Child’s Pugh (CP) B, median time to progression was 4.2 months and overall survival was 9.2 months. There was no difference in the tolerability of sorafenib in patients with CP Class A or B suggesting that this therapy, or its use in combination, would be tolerated for patients with more advanced liver disease [Abou-Alfa et al. 2006]. Subsequently published work suggests that dosing of sorafenib should be reduced in patients with bilirubin >1.5 times upper limit of normal [Miller et al. 2009]. This dosing recommendation was not adopted in a small retrospective study of sorafenib in 59 patients, 23 of whom were classified at CP B and 10 of whom were CP C with unresectable HCC. Primary endpoints were time to progression and overall survival among patients with different CP scores. Median survival time for patients with CP A, B, and C were 8.3 months, 4.3 months, and 1.5 months, respectively (p = 0.0001). When the authors grouped the patients according to Barcelona Clinic Liver Cancer (BCLC) staging classification, survival was 10.2 months for patients with stage B–C disease and 1.5 months for patients with stage D disease. Based on this, the authors reasonably concluded that there is no benefit to systemic therapy in patients with very advanced disease [Pinter et al. 2009]. The use of sorafenib in patients with decompensated liver disease needs to be studied in a prospective manner to better assess its efficacy.
Overall, sorafenib is usually well tolerated. Major side effects include hand–foot syndrome (5–8% in the US study and 11.4 % in the Asian study) fatigue (8–10%), and diarrhea (9%). While there was no major bleeding in Abou Alfa’s phase II study and no significant increase in grade 3/4 bleeding in SHARP, Pinter reported a 7% rate of severe GI bleeding, although this complication only developed in patients with CP B or C disease.
The identification of sorafenib and its role in the treatment of HCC marks a major advance in the field as the first targeted therapy directed against clear molecular pathways in HCC which has shown a survival benefit in randomized controlled trials. Sorafenib is now approved for the treatment of advanced HCC in the US, and its role in treatment is being studied in different contexts. For instance, it is being evaluated in patients undergoing locoregional treatment as well as in patients undergoing surgical resection and transplant. It is also being studied in the neoadjuvant setting to attempt to downstage patients prior to surgery. The potential roles for this drug remain a dynamic frontier for those studying HCC therapy, and its success to date underscores the need to develop additional targeted agents that play a role in HCC development.
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody directed against VEGF. It is approved for the treatment of several malignancies in the US including non-small-cell lung cancer, breast and colon cancer. Bevacizumab has been studied as a single agent in the treatment of HCC. In a multicenter phase II study of 46 patients with compensated liver disease and unresectable HCC, bevacizumab led to 6-month progression-free survival in 65% of patients, with 13% experiencing a partial response to treatment. Median progression-free survival was 6.9 months and overall survival was 53% at 1 year. A major side effect of bevacizumab is bleeding; the bleeding rate in this study was 11% [Siegel et al. 2008a]. Bevacizumab has also been studied in conjunction with oxaliplatin and gemcitabine (GEMOX) for treatment of unresectable HCC. In a phase II study of 33 patients, almost half of whom had metastatic disease, treatment with bevacizumab and GEMOX resulted in a 20% response rate with an overall median survival of 9.6 months. While there were no patients with a complete response, 27% had stable disease. It should be noted, however, that treatment was discontinued in 36% of patients and it is not clear from these results that bevacizumab adds efficacy to GEMOX alone [Zhu et al. 2006].
Erlotinib
Erlotinib is a small molecule inhibitor which targets the EGFR tyrosine kinase. Erlotinib has been studied as a single agent in HCC in two US phase II trials at a dose of 150 mg daily. The first study included 38 patients with unresectable HCC, almost half of whom had been treated with prior chemotherapy. Thirty-two per cent of patients were progression-free at 6 months and median overall survival was 13 months [Philip et al. 2005]. When studied in 40 patients with Childs A or B cirrhosis and advanced HCC, there were no complete or partial responses, but overall survival was 10.8 months, suggesting a possible benefit to the use of this therapy [Thomas et al. 2007]. Recently, Thomas and colleagues evaluated the combination of erlotinib with bevacizumab in a phase II study of 40 patients. This combination yielded a median overall survival of 68 weeks [Thomas et al. 2009]. Side effects of this regimen include gastrointestinal bleeding (12.5%) and fatigue (20%).
Cetuximab
In contrast to erlotinib, cetuximab is a monoclonal antibody against the EGF receptor targeting the extracellular receptor-binding site. It has been studied alone and in combination with other agents in HCC. In one phase II trial, it was given to 30 patients with unresectable HCC for a period of 6 weeks. While the regimen was well tolerated, in contrast to erlotinib, no objective tumor responses were seen [Zhu et al. 2007]. In 45 treatment-naïve patients with advanced HCC, the combination of gemcitabine and oxaliplatin with cetuximab was associated with progression-free survival of 4.7 months, overall survival of 9.5 months and a 40% 1-year survival rate [Asnacios et al. 2008]. Given the efficacy of the GEMOX combination without the additional agent, and the lack of convincing evidence as monotherapy, the efficacy of cetuximab remains uncertain.
PI3K/Akt/mTOR pathway
EGF and the insulin growth factor (IGF) signaling pathways activate a protein known as PI3K, which in turn activates Akt, which then activates mTOR (mammalian target of rapamycin). mTOR is a key regulator of cell growth [Villanueva et al. 2008]. Rapamycin (Sirolimus) is an mTOR inhibitor that has been shown to have antitumor properties and can also be used an immunosuppressive agent post-transplant [Heuer et al. 2009; Nocera et al. 2008; Toso et al. 2007]. In a retrospective study of 73 patients who underwent OLT for HCC outside of Milan criteria, those who received rapamycin had better survival than those who were given tacrolimus-based immunosuppression [Zhou et al. 2008]. Retrospective data from the University of Colorado suggest that patients who were transplanted for HCC and put on sirolimus- based immunosuppression immediately following transplant had better 1- and 5-year overall survival rates compared to patients who received tacrolimus- or cyclosporin-based regimens (95.5% and 78.8% compared to 83% and 62%, respectively, although these were not statistically significant) [Zimmerman et al. 2008]. This suggests that sirolimus may be beneficial as first-line immunosuppression for patients transplanted for HCC. The use of sirolimus is associated with an increased risk of hepatic artery thrombosis and poor wound healing, primarily in the immediate post-transplant period, which limits its use as initial immunosuppression.
Rapamycin has also been studied outside of transplantation. In a small pilot study of 21 patients with either HCC or cholangiocarcinoma, six patients with HCC had either stable disease or had a partial remission [Rizell et al. 2008]. Clearly, further studies need to be done to assess the role of rapamycin in the treatment of HCC, both in the pre- and post-transplant setting. Everolimus is another mTOR inhibitor that has been shown to have activity against HCC in xenografts and is now being studied in phase II trials in metastatic disease [Huynh et al. 2008]. Everolimus has also been studied with sorafenib treatment with promising early results [Huynh et al. 2009].
Locoregional therapy
Despite the development of molecular targeted therapies, the two most effective treatment approaches to HCC are still liver resection and transplantation. A third treatment commonly used in HCC is locoregional therapy. Locoregional therapy refers to a variety of treatments directed at the tumor, including ablation and transarterial chemoembolization (TACE). Ablation is usually accomplished now with radiofrequency ablation (RFA) rather than percutaneous ethanol ablation (PEI). RFA has been shown to be more effective in tumors <4 cm compared with PEI [Lin et al. 2004]. RFA and PEI have been compared in randomized studies and RFA has been associated with improved recurrence-free survival rates of 86% at 1 year and 64% at 2 years, compared to 77% and 43% respectively, for the PEI group [Lencioni et al. 2003]. A recently published meta-analysis also demonstrated superiority of RFA compared with PEI, with better tumor response rates, lower recurrence rates and better overall survival [Orlando et al. 2009].
TACE takes advantage of the dominant arterial blood supply of HCC. While the normal liver enjoys dual blood supply from both the hepatic artery and the portal vein, tumors preferentially derive their blood supply from the hepatic artery. TACE typically involves the injection of a chemotherapeutic agent such as doxorubicin or cisplatin suspended in a contrast medium such as lipiodol or gelfoam in the hepatic artery or arteries supplying the tumor. The goal of TACE is to disrupt the blood supply to the tumor while instilling chemotherapeutic agents directly into the tumor.
TACE is typically used in patients with larger or multifocal tumors, preserved liver function, and asymptomatic cancer with the absence of vascular invasion. TACE results in tumor necrosis in 30–50% of patients and is generally well tolerated, with adverse events in the range of 10% [Bruix et al. 2006; Forner et al. 2006]. In a large prospective study of 8510 patients, TACE was associated with an 82% 1 year survival and a 47% 2 year survival [Bruix and Llovet, 2003; Burrel et al. 2003; Llovet and Bruix, 2003]. A meta-analysis of seven randomized controlled trials comparing TACE to conservative management and/or percutaneous therapies showed that TACE improved 2-year survival compared to control subjects in a carefully selected patient population (odds ratio 0.53; CI: 0.32–0.89) [Bruix et al. 2004]. The combination of TACE and PEI has been shown to be superior to single modality treatment with either TACE or PEI. In two small randomized control trials, the survival rates of TACE/REI combination therapy were better than those of PEI [Koda et al. 2001] and TACE alone [Kamada et al. 2002].
TACE may also play a role in downsizing tumors prior to surgery. Yao and his colleagues at University of California San Francisco (UCSF) report on a total of 61 patients who underwent downsizing of their tumors with a 70% success rate. Fifty-seven per cent of those patients underwent OLT, and there were no cases of tumor recurrence at a median follow-up post-transplant of 25 months [Yao et al. 2008]. Other studies support the use of TACE as a means of downsizing tumors to make transplant possible, but additional work is needed to clarify the outcomes of these patients [Chapman et al. 2008; Otto et al. 2006]. Results from randomized trials, however, are limited. In a study of 108 patients with HBV and resectable HCC, patients were randomized to preoperative TACE or not. In this small study, there was no significant difference in disease recurrence rates or disease-free survival between the two groups [Zhou et al. 2009b].
To summarize, TACE is established as primary therapy in patients with unresectable HCC and no vascular invasion or disease outside the liver. It is also used for patients who are deemed appropriate transplant candidates as a means of stabilizing their disease while on the waiting list. Finally, TACE is used in patients outside of transplant criteria as a way of downsizing their disease burden, with the hope that they will meet criteria for transplant, although the data supporting this practice is limited [Vogl et al. 2008]. Other forms of locoregional therapy are also currently in use, including bland embolization and radiolabeled and drug-eluting beads. All are undergoing continued investigation [Poon et al. 2002].
Surgery
Surgery encompasses transplant as well as resection. Hepatic resection is the treatment of choice for noncirrhotic patients with limited disease (Figure 2). This group accounts for <5% of patients in Western countries, but almost 40% of patients in countries where HBV is endemic, since cirrhosis is not a prerequisite for developing HCC in the context of HBV [Bolondi et al. 2001]. Transplantation is the treatment of choice for patients with cirrhosis who meet Milan criteria, namely: (1) single nodule <5 cm or (2) a maximum of three nodules, each <3 cm, and no gross vascular invasion [Mazzaferro et al. 1996]. These criteria are also endorsed by the United Network for Organ Sharing (UNOS) as a way to determine which patients are eligible for increased priority for transplant [Sala et al. 2004]. Under the current system, those who are within Milan criteria are eligible for Model for End Stage Liver Disease (MELD) exception points of 22. This often helps patients with HCC and otherwise compensated liver disease (i.e. low MELD scores) get transplanted. Based on these criteria, transplantation has resulted in 5-year survival rates of >70% and recurrence rates under 15% for patients with HCC [Siegel et al. 2008b; Shetty et al. 2004].
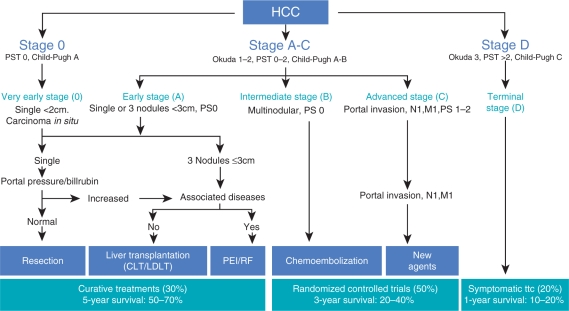
Figure 2.
The Barcelona Clinic Liver Cancer (BCLC) staging and treatment schedule. Reprinted with permission of John Wiley & Sons, Inc. [Llovet 2005]. PST, performance status test; CLT, cadaveric liver transplantation; LDLT, living donor liver transplantation; PEI, percutaneous ethanol injection; RF, radiofrequency thermal ablation; ttc, treatment.
Some centers feel these criteria are too restrictive. The group at the UCSF has published similar results in patients with more advanced disease. They have established the UCSF criteria, namely (1) single lesion <6.5 cm or (2) up to three lesions, each <3 cm, with total size <8 cm [Duffy et al. 2007; Yao et al. 2001]. UCSF as well as others in the transplant community have advocated that these more lenient criteria be utilized when making a decision regarding transplant [Yao, 2008; Yao et al. 2008].
Mazzaferro and colleagues studied data from 1556 patients transplanted for HCC in an effort to design a prognostic model that predicted survival after transplant based on objective criteria such as tumor burden (number and size of nodules) and presence or absence of vascular invasion as opposed to an ‘all-or-nothing’ model where criteria are met or not. They found that for a select group of patients outside of Milan criteria, without microvascular invasion, who fell within ‘up to seven’ criteria [meaning the size of the largest tumor (in cm) plus the number of nodules present had to sum to 7 cm or less], had a 5-year survival rate of 71%, which is comparable to the survival rate of patients within Milan criteria [Mazzaferro et al. 2009]. Although additional refinement may be useful in improving the accuracy of the model, these data suggest that moving beyond the Milan criteria to a less restrictive model is feasible. There is currently no consensus on the role of neoadjuvant therapy or adjuvant therapy pre- or postsurgery, although both are under active investigation.
Living donor liver transplantation (LDLT) is an attractive option for patients with HCC who have compensated liver disease and do not have a MELD score high enough obtain a deceased donor graft. Although single-center experiences with LDLT for patients with HCC are promising, the number of patients studied is small and data are limited [Concejero et al. 2008; Pandey et al. 2008]. Data from the multicenter Adult to Adult Living Donor Liver Transplantation (A2ALL) group suggests higher recurrence rates for patients after LDLT than deceased donor liver transplant (DDLT) [Fisher et al. 2007]. LDLT for patients with HCC beyond Milan criteria is associated with poor disease-free recurrence rates and is therefore not currently standard practice [Fouzas et al. 2008; Sotiropoulos et al. 2008a, 2008b] although data from UCSF in a small number of patients are promising [Jiang et al. 2008]. Lee and colleagues report on 221 patients undergoing LDLT for HCC; only 15% were within Milan or UCSF criteria. Their expanded criteria (patients had primary tumor size <5 cm, total tumor number <6, and no vascular invasion), resulted in greater discriminatory power than UCSF or Milan criteria, thus increasing the number of patients eligible for LDLT and more accurately identifying which patients would benefit from this approach [Lee et al. 2008].
Current treatment approach
The current approach to treatment of HCC depends on both the severity of liver disease and the extent of the tumor. The European Association for the Study of Liver Disease (EASL) also supports incorporating patients’ performance status in the treatment algorithm [Bruix et al. 2001]. The Barcelona Clinic Liver Cancer (BCLC) staging classification system is most commonly used, in conjunction with CP score to determine the optimal therapeutic modality [Llovet et al. 1999]. The algorithm for therapy is shown in Figure 2. Patients with stage 0 disease, very early HCC with tumor nodule <2 cm, and no manifestations of portal hypertension are typically good candidates for resection. Patients with cirrhosis, a single tumor <5 cm in size, or three nodules, each less than 3 cm in size should be evaluated for transplantation. For those patients who have a contraindication to transplant, locoregional therapies are recommended. These modalities are also used to prevent tumor progression while on the transplant waiting list. Stage B represent those patients with multinodular disease, for whom TACE is first-line therapy. As outlined earlier, there are data to suggest that patients in this category may be downsized to within Milan criteria and thus be better candidates for transplant. Stage C includes those patients with portal vein invasion or disease outside the liver without hepatic decompensation who could receive sorafenib. For stage D patients with decompensated cirrhosis, palliative treatment is still recommended.
Genetic profiling
Currently, there is no one molecular framework for classifying HCC. However, much effort is being directed at correlating genetics with prognosis and outcome. Lee and Thorgeirsson report on 91 HCC samples analyzed for over 4000 genes. They clustered the tumors in two categories based on survival, and found that genes associated with anti-apoptosis and cell proliferation profiles were more prevalent in tumors of patients with poorer survival [Lee and Thorgeirsson, 2004].
Genetic profiling is also an attractive tool to identify HCC tumors that are more likely to recur. Hoshida and colleagues attempted to find a gene expression pattern associated with survival in patients with resected HCC. Although they failed to detect a correlation between gene expression in the tumor and survival or recurrence, by examining gene expression in liver tissue surrounding the tumor, they were able to show an association between a 132 gene profile and both late recurrence of tumors and survival [Hoshida et al. 2009, 2008]. Other studies have looked at patients with HBV and HCC and identified an SP1 transcription factor, and peroxisome proliferator oxidative receptor α (PPAR α) as common regulators of genes that differed between patients with HCC recurrence and those without [Woo et al. 2008].
Genetic profiling may also provide information on potential therapeutic targets for HCC. Zhou and colleagues studied 528 HCC tumors and looked at the expression of PTEN, pAkt, p27, and pS6, all of which function in the mTOR pathway. The expression of each of these proteins was shown to be an independent negative prognostic factor for HCC [Zhou et al. 2009a].
Future directions
Studying HCC is challenging due to the diverse patient population involved, different etiologies of liver disease, and variety of potential therapeutic modalities (locoregional, resection, transplantation, and increasingly molecularly targeted treatments). There are several challenges facing the hepatology, transplant and oncologic community. First, additional work is required to clarify the molecular pathogenesis of HCC and identify key markers for therapeutic intervention. The development of sorafenib underscores the potential to target additional receptors and other mediators in tumorogenic pathways. Second, we need to ensure that various therapies are studied in combination with each other and also in succession. Specifically, the role of sorafenib as adjunctive therapy needs to be evaluated both pre- and postsurgery, as well as with locoregional therapies. For instance, there is an ongoing prospective, randomized, phase III trial looking at the role of sorafenib in patients with HCC who are not transplant candidates receiving TACE therapy. Another trial is assessing sorafenib in high-risk post-resection patients in a large randomized trial. Our own center is piloting sorafenib in the post-transplant setting to assess safety when given with immunosuppressants. Third, study design needs to be critically evaluated, particularly in the age of molecular therapies. The AASLD has recently convened a panel of multidisciplinary experts to comment on the design of clinical trials for HCC. This group suggested that randomized phase II trials, designed to determine antitumor activity of a particular agent, be encouraged and that time to progression, not response rate, might be a more useful endpoint in these trials. This thinking is underscored by data that show that survival advantages can be seen in the absence of significant tumor response rates (as seen in the SHARP study) and that response rate may not capture the benefit of a molecular therapy as it would with an intervention such as TACE or RFA [Llovet et al. 2008a]. Finally as transplant remains the primary method of curing HCC and in the West, every effort needs to be made to improve our screening for HCC and streamlining the process of referral of eligible HCC patients to a transplant center.
Summary
The treatment of HCC is complicated by the wide variety of underlying liver diseases associated with the development of this tumor as well as the number of therapeutic modalities available to patients. The identification of sorafenib and the survival benefit it confers to patients with unresectable HCC represents a new era in the treatment of HCC. Future studies need to focus on additional molecular targets as well as determining the optimal timing and combination of treatment modalities to maximize patient outcome. Liver transplantation remains the primary potential cure for this tumor in the West, underscoring the need for vigilant screening and effective referral to transplant centers.
Acknowledgments
Supported in part by a K12 award from the National Institutes of Health (KL2 RR024157-03) and the Steven J. Levinson Medical Research Foundation.
Conflict of interest statement
None declared.
References
- Abou-Alfa G.K., Schwartz L., Ricci S., Amadori D., Santoro A., Figer A., et al. (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 4293–4300 [DOI] [PubMed] [Google Scholar]
- Asnacios A., Fartoux L., Romano O., Tesmoingt C., Louafi S.S., Mansoubakht T., et al. (2008) Gemcitabine plus oxaliplatin (Gemox) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer 112: 2733–2739 [DOI] [PubMed] [Google Scholar]
- Bolondi L., Sofia S., Siringo S., Gaiani S., Casali A., Zironi G., et al. (2001) Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 48: 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F.X., Ribes J., Cleries R., Diaz M. (2005) Epidemiology of hepatocellular carcinoma. Clin Liver Dis 9: 191–211, v [DOI] [PubMed] [Google Scholar]
- Bruix J., Hessheimer A.J., Forner A., Boix L., Vilana R., Llovet J.M. (2006) New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 25: 3848–3856 [DOI] [PubMed] [Google Scholar]
- Bruix J., Llovet J.M. (2003) HCC surveillance: who is the target population? Hepatology 37: 507–509 [DOI] [PubMed] [Google Scholar]
- Bruix J., Sala M., Llovet J.M. (2004) Chemoembolization for hepatocellular carcinoma. Gastroenterology 127: S179–188 [DOI] [PubMed] [Google Scholar]
- Bruix J., Sherman M. (2005) Management of hepatocellular carcinoma. Hepatology 42: 1208–1236 [DOI] [PubMed] [Google Scholar]
- Bruix J., Sherman M., Llovet J.M., Beaugrand M., Lencioni R., Burroughs A.K., et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35: 421–430 [DOI] [PubMed] [Google Scholar]
- Burrel M., Llovet J.M., Ayuso C., Iglesias C., Sala M., Miquel R., et al. (2003) MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology 38: 1034–1042 [DOI] [PubMed] [Google Scholar]
- Chapman W.C., Majella Doyle M.B., Stuart J.E., Vachharajani N., Crippin J.S., Anderson C.D., et al. (2008) Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 248: 617–625 [DOI] [PubMed] [Google Scholar]
- Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25–34 [DOI] [PubMed] [Google Scholar]
- Concejero A., Chen C.L., Wang C.C., Wang S.H., Lin C.C., Liu Y.W., et al. (2008) Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation 85: 398–406 [DOI] [PubMed] [Google Scholar]
- De Ledinghen V., Laharie D., Lecesne R., Le Bail B., Winnock M., Bernard P.H., et al. (2002) Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. Eur J Gastroenterol Hepatol 14: 159–165 [DOI] [PubMed] [Google Scholar]
- Duffy J.P., Vardanian A., Benjamin E., Watson M., Farmer D.G., Ghobrial R.M., et al. (2007) Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 246: 502–509, discussion 509–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H.B., Marrero J.A., Rudolph L., Reddy K.R. (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134: 1752–1763 [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Mason A.C. (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340: 745–750 [DOI] [PubMed] [Google Scholar]
- Fisher R.A., Kulik L.M., Freise C.E., Lok A.S., Shearon T.H., Brown Jr R.S., et al. (2007) Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 7: 1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner A., Hessheimer A.J., Isabel Real M., Bruix J. (2006) Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol 60: 89–98 [DOI] [PubMed] [Google Scholar]
- Fouzas I., Sotiropoulos G.C., Lang H., Nadalin S., Beckebaum S., Sgourakis G., et al. (2008) Living donor liver transplantation for hepatocellular carcinoma in patients exceeding the UCSF criteria. Transplant Proc 40: 3185–3188 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Heuer M., Benko T., Cicinnati V.R., Kaiser G.M., Sotiropoulos G.C., Baba H.A., et al. (2009) Effect of low-dose rapamycin on tumor growth in two human hepatocellular cancer cell lines. Transplant Proc 41: 359–365 [DOI] [PubMed] [Google Scholar]
- Hoshida Y., Villanueva A., Kobayashi M., Peix J., Chiang D.Y., Camargo A., et al. (2008) Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 359: 1995–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y., Villanueva A., Llovet J.M. (2009) Molecular profiling to predict hepatocellular carcinoma outcome. Expert Rev Gastroenterol Hepatol 3: 101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H., Chow K.P., Soo K.C., Toh H.C., Choo S.P., Foo K.F., et al. (2008) Rad001 (Everolimus) inhibits tumor growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med 7: 1371–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H., Ngo V.C., Koong H.N., Poon D., Choo S.P., Thng C.H., et al. (2009) Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.Z., Yan L.N., Wen T.F., Li B., Zeng Y., Zhao J.C., et al. (2008) University of California at San Francisco criteria can be applied to living donor liver transplantation for hepatocellular carcinoma: single-center preliminary results in 27 patients. Transplant Proc 40: 1476–1480 [DOI] [PubMed] [Google Scholar]
- Kamada K., Kitamoto M., Aikata H., Kawakami Y., Kono H., Imamura M., et al. (2002) Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg 184: 284–290 [DOI] [PubMed] [Google Scholar]
- Koda M., Murawaki Y., Mitsuda A., Oyama K., Okamoto K., Idobe Y., et al. (2001) Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer 92: 1516–1524 [DOI] [PubMed] [Google Scholar]
- Lee J.S., Thorgeirsson S.S. (2004) Genome-scale profiling of gene expression in hepatocellular carcinoma: classification, survival prediction, and identification of therapeutic targets. Gastroenterology 127: S51–55 [DOI] [PubMed] [Google Scholar]
- Lee S.G., Hwang S., Moon D.B., Ahn C.S., Kim K.H., Sung K.B., et al. (2008) Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 14: 935–945 [DOI] [PubMed] [Google Scholar]
- Lencioni R.A., Allgaier H.P., Cioni D., Olschewski M., Deibert P., Crocetti L., et al. (2003) Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228: 235–240 [DOI] [PubMed] [Google Scholar]
- Libbrecht L., Bielen D., Verslype C., Vanbeckevoort D., Pirenne J., Nevens F., et al. (2002) Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl 8: 749–761 [DOI] [PubMed] [Google Scholar]
- Lin S.M., Lin C.J., Lin C.C., Hsu C.W., Chen Y.C. (2004) Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology 127: 1714–1723 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Bru C., Bruix J. (1999) Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 19: 329–338 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Bruix J. (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37: 429–442 [DOI] [PubMed] [Google Scholar]
- Llovet J.M. (2005) Updated treatment approach to hepatocellular carcinoma. J Gastroenterology 40: 225–35 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Bruix J. (2008) Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48: 1312–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J.M., Chen Y., Wurmbach E., Roayaie S., Fiel M.I., Schwartz M., et al. (2006) A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 131: 1758–1767 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Di Bisceglie A.M., Bruix J., Kramer B.S., Lencioni R., Zhu A.X., et al. (2008a) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100: 698–711 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. (2008b) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390 [DOI] [PubMed] [Google Scholar]
- Marrero J.A., Feng Z., Wang Y., Nguyen M.H., Befeler A.S., Roberts L.R., et al. (2009) Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 137: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L., et al. (2009) Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10: 35–43 [DOI] [PubMed] [Google Scholar]
- Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334: 693–699 [DOI] [PubMed] [Google Scholar]
- Miller A.A., Murry D.J., Owzar K., Hollis D.R., Kennedy E.B., Abou-Alfa G., et al. (2009) Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 27: 1800–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon W.S., Rhyu K.H., Kang M.J., Lee D.G., Yu H.C., Yeum J.H., et al. (2003) Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol 16: 552–557 [DOI] [PubMed] [Google Scholar]
- Nocera A., Andorno E., Tagliamacco A., Morelli N., Bottino G., Ravazzoni F., et al. (2008) Sirolimus therapy in liver transplant patients: an initial experience at a single center. Transplant Proc 40: 1950–1952 [DOI] [PubMed] [Google Scholar]
- Orlando A., Leandro G., Olivo M., Andriulli A., Cottone M. (2009) Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol 104: 514–524 [DOI] [PubMed] [Google Scholar]
- Otto G., Herber S., Heise M., Lohse A.W., Monch C., Bittinger F., et al. (2006) Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 12: 1260–1267 [DOI] [PubMed] [Google Scholar]
- Ozanne B.W., Spence H.J., McGarry L.C., Hennigan R.F. (2007) Transcription factors control invasion: AP-1 the first among equals. Oncogene 26: 1–10 [DOI] [PubMed] [Google Scholar]
- Pandey D., Wai C.T., Lee K.H., Tan K.C. (2008) Living donor liver transplantation for hepatocellular carcinoma: a single centre experience. Indian J Gastroenterol 27: 148–152 [PubMed] [Google Scholar]
- Philip P.A., Mahoney M.R., Allmer C., Thomas J., Pitot H.C., Kim G., et al. (2005) Phase II study of erlotinib (Osi-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23: 6657–6663 [DOI] [PubMed] [Google Scholar]
- Pinter M., Sieghart W., Graziadei I., Vogel W., Maieron A., Konigsberg R., et al. (2009) Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist 14: 70–76 [DOI] [PubMed] [Google Scholar]
- Pisani P., Bray F., Parkin D.M. (2002) Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer 97: 72–81 [DOI] [PubMed] [Google Scholar]
- Poon R.T., Fan S.T., Tsang F.H., Wong J. (2002) Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon’s perspective. Ann Surg 235: 466–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizell M., Andersson M., Cahlin C., Hafstrom L., Olausson M., Lindner P. (2008) Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol 13: 66–70 [DOI] [PubMed] [Google Scholar]
- Rode A., Bancel B., Douek P., Chevallier M., Vilgrain V., Picaud G., et al. (2001) Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr 25: 327–336 [DOI] [PubMed] [Google Scholar]
- Sala M., Varela M., Bruix J. (2004) Selection of candidates with HCC for transplantation in the meld era. Liver Transpl 10: S4–9 [DOI] [PubMed] [Google Scholar]
- Schiffer E., Housset C., Cacheux W., Wendum D., Desbois-Mouthon C., Rey C., et al. (2005) Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 41: 307–314 [DOI] [PubMed] [Google Scholar]
- Shetty K., Timmins K., Brensinger C., Furth E.E., Rattan S., Sun W., et al. (2004) Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl 10: 911–918 [DOI] [PubMed] [Google Scholar]
- Siegel A.B., Cohen E.I., Ocean A., Lehrer D., Goldenberg A., Knox J.J., et al. (2008a) Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 26: 2992–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A.B., McBride R.B., El-Serag H.B., Hershman D.L., Brown Jr R.S., Renz J.F., et al. (2008b) Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998–2002. Am J Gastroenterol 103: 120–127 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos G.C., Kaiser G.M., Lang H., Molmenti E.P., Beckebaum S., Fouzas I., et al. (2008a) Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc 40: 3194–3195 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos G.C., Lang H., Saner F.H., Beckebaum S., Wandelt M., Molmenti E.P., et al. (2008b) Long-term results after liver transplantation with ‘livers that nobody wants’ within Eurotransplant: a center’s experience. Transplant Proc 40: 3196–3197 [DOI] [PubMed] [Google Scholar]
- Thomas M.B., Chadha R., Glover K., Wang X., Morris J., Brown T., et al. (2007) Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer 110: 1059–1067 [DOI] [PubMed] [Google Scholar]
- Thomas M.B., Morris J.S., Chadha R., Iwasaki M., Kaur H., Lin E., et al. (2009) Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol 27: 843–850 [DOI] [PubMed] [Google Scholar]
- Toso C., Meeberg G.A., Bigam D.L., Oberholzer J., Shapiro A.M., Gutfreund K., et al. (2007) De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation 83: 1162–1168 [DOI] [PubMed] [Google Scholar]
- Villanueva A., Chiang D.Y., Newell P., Peix J., Thung S., Alsinet C., et al. (2008) Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 135: 1972–1983, 1983 e1971–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T.J., Naguib N.N., Nour-Eldin N.E., Rao P., Emami A.H., Zangos S., et al. (2008) Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol [In press] [DOI] [PubMed] [Google Scholar]
- Woo H.G., Park E.S., Cheon J.H., Kim J.H., Lee J.S., Park B.J., et al. (2008) Gene Expression-based recurrence prediction of hepatitis B virus-related human hepatocellular carcinoma. Clin Cancer Res 14: 2056–2064 [DOI] [PubMed] [Google Scholar]
- Yao F.Y. (2008) Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant 8: 1982–1989 [DOI] [PubMed] [Google Scholar]
- Yao F.Y., Ferrell L., Bass N.M., Watson J.J., Bacchetti P., Venook A., et al. (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33: 1394–1403 [DOI] [PubMed] [Google Scholar]
- Yao F.Y., Kerlan Jr R.K., Hirose R., Davern 3rd T.J., Bass N.M., Feng S., et al. (2008) Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 48: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiji H., Kuriyama S., Yoshii J., Yamazaki M., Kikukawa M., Tsujinoue H., et al. (1998) Vascular endothelial growth factor tightly regulates in vivo development of murine hepatocellular carcinoma cells. Hepatology 28: 1489–1496 [DOI] [PubMed] [Google Scholar]
- Zhang B.H., Yang B.H., Tang Z.Y. (2004) Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130: 417–422 [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang Z., Wu Z.Q., Qiu S.J., Yu Y., Huang X.W., et al. (2008) Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc 40: 3548–3553 [DOI] [PubMed] [Google Scholar]
- Zhou L., Huang Y., Li J., Wang Z. (2009a) The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol [In press] [DOI] [PubMed] [Google Scholar]
- Zhou W.P., Lai E.C., Li A.J., Fu S.Y., Zhou J.P., Pan Z.Y., et al. (2009b) A Prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg 249: 195–202 [DOI] [PubMed] [Google Scholar]
- Zhu A.X. (2008) Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer 112: 250–259 [DOI] [PubMed] [Google Scholar]
- Zhu A.X., Blaszkowsky L.S., Ryan D.P., Clark J.W., Muzikansky A., Horgan K., et al. (2006) Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 1898–1903 [DOI] [PubMed] [Google Scholar]
- Zhu A.X., Stuart K., Blaszkowsky L.S., Muzikansky A., Reitberg D.P., Clark J.W., et al. (2007) Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 110: 581–589 [DOI] [PubMed] [Google Scholar]
- Zimmerman M.A., Trotter J.F., Wachs M., Bak T., Campsen J., Skibba A., et al. (2008) Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 14: 633–638 [DOI] [PubMed] [Google Scholar]