Abstract
Background: Mycophenolate mofetil (MMF, CellCept®) has been utilized as an antirejection agent in transplant recipients and in patients with myriad autoimmune disorders including multiple sclerosis (MS). Objective: To investigate radiographic and clinical safety involving monotherapy use of daily oral MMF (1 g b.i.d.) versus weekly intramuscular interferon beta 1a (Avonex® at 30 mcg) in relapsing–remitting MS (RRMS). Methods: We organized a randomized, serial, 6-monthly, MRI-blinded, parallel-group multicenter pilot study to determine the safety of MMF versus interferon beta monotherapy in 35 untreated patients with RRMS, all of whom exhibited evidence of gadolinium (Gd) enhancement on a screening MRI of the brain. The primary outcome was the reduction in the cumulative mean number of combined active lesions (CAL), new Gd-enhancing lesions, and new T2 lesions on MRI analyses. Results: Both interferon beta and MMF appeared safe and well tolerated in the majority of patients. There was no difference between MMF therapy and the standard regimen of interferon beta therapy on the primary safety MRI endpoints of the study. However, the MMF group showed a trend toward a lower accumulation of combined active lesions, CAL, Gd and T2 lesions when compared with interferon beta treated patients. Conclusions: The results from this pilot study suggest that the application of MMF monotherapy in MS deserves further exploration.
Keywords: mycophenolate mofetil, CellCept, interferon beta 1a, MRI activity, immunosuppression, relapsing–remitting multiple sclerosis
First-line disease modifying therapy (DMT) for multiple sclerosis (MS) has included the administration of parenteral agents such as interferon beta or glatiramer acetate [Corboy et al. 2003; Goodin et al. 2002]. Over a long period of utilization, from clinical trials to post-marketing surveillance, these agents have been demonstrated to be well tolerated and exquisitely safe in the majority of treated patients. Despite these important safety merits, the efficacy of these drugs, while more than adequate for some patients, has been insufficient for others, particularly over time. Furthermore, adherence to parenteral forms of MS DMTs has been a challenge for many patients, which is a consideration that has clear implications for the effectiveness of treatment [Treadway et al. 2009].
Modest efficacy, adherence problems and injection-related adverse events have catalyzed a series of investigations aimed at the identification of an effective oral therapy for MS. There are now five agents in phase III clinical trials that appear promising in providing superior efficacy, greater convenience of oral administration, and improved adherence for our MS patients. These include cladribine, FTY-720, dimethylfumarate (BG-12), laquinimod and teriflunomide [Cohen, 2009; Menge et al. 2008]. The latter agent is related to the approved drug leflunomide, which has long been utilized for the treatment of rheumatoid arthritis. Teriflunomide is an inhibitor of dihydro-orate dehydrogenase, a key enzyme in the synthesis of pyrimidines [O’Connor et al. 2006].
Compared with the proprietary oral agents under development, mycophenolate mofetil (MMF) is a readily available and cost effective therapy. MMF would be of potentially great interest in MS therapeutics given that this agent exerts pleiotropic effects on T and B cells and macrophages, all key cellular participants involved in the orchestration of CNS tissue injury [Frohman et al. 2006, 2004]. Similar to teriflunomide, MMF is a DNA base synthesizing enzyme system inhibitor. Specifically, MMF is a selective inhibitor of inosine 5′-monophosphate dehydrogenase (IMD) type II, which is a potent immunosuppressant, principally used in transplant medicine as an antirejection agent [Jonsson and Carlsten, 2002]. IMD is responsible for the de novo synthesis of the purine nucleotide guanine within activated lymphocytes, and macrophages, without affecting purine salvage pathways. MMF is the prodrug of mycophenolic acid (MPA), which has been shown to be on the order of five times more potent than MMF as an inhibitor of the type II isoform of IMD. This observation is relevant in that the type II isoform is predominant in lymphocytes, whereas the type I variety is the principal form in all other cell types [Allison and Eugi, 1993; Allison et al. 1993].
MMF has been shown to exert a number of immunomodulatory activities that may be useful in the treatment of immune-mediated diseases. For instance, MMF exhibits the capability to suppress lymphocyte proliferation and the expression of T-cell surface antigens in whole blood lymphocyte analysis derived from treated allograft recipients [Barten et al. 2002a, 2002b]. MPA has been shown to inhibit interferon gamma (IFN-γ) and lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) and nitric oxide [Miljkovic et al. 2002]. This latter activity may correspondingly confer therapeutic benefits for patients with MS, given that the disease mechanism appears to involve a skewing or bias toward pro-inflammatory immune responses, in part characterized by the inappropriate elaboration of IL-6 and nitric oxide [Noseworthy et al. 2000]. Further, mononuclear cell trafficking from blood into the brain can be modified by MMF by reducing the expression of vascular cell adhesion molecule 1 [Blaheta et al. 1999]. Humorally MMF effectively suppresses anti-blood type IgG antibodies in patients receiving ABO mismatched renal transplants [Ishida et al. 2002].
Preliminary studies have shown good tolerability and safety when MMF is utilized as monotherapy or in combination with interferons or glatiramer acetate [Vermersch et al. 2007; Frohman et al. 2004]. We now report the results of a randomized, blinded, parallel-group, head-to-head pilot study of MMF compared with weekly intramuscular interferon beta 1a, in untreated RRMS patients who presented with evidence of gadolinium (Gd)-enhancing MRI brain lesions. The principal aim of our pilot study was to assess 6-month safety and potential efficacy of MMF (CellCept) versus interferon beta-1a (Avonex) (6.0 Million international units (MIU) administered i.m. each week) therapy, by comparing month-to-month changes of two inflammatory MRI measures – Gd-enhancing and T2 lesion metrics.
Methods
Patient selection
Patients were enrolled and randomized from three centers: University of Texas Southwestern Medical Center at Dallas, the Michigan Institute for Neurological Disorders and the Jacobs Neurological Institute, State University of New York, Buffalo, NY. All subjects signed informed consent prior to any study-related assessments and the study was approved by each center’s local Institutional Review Board.
Inclusion criteria
Patients were eligible for this study if they were diagnosed with clinically definite MS according to McDonald criteria [Polman et al. 2005], were aged 18–55 inclusive, had a relapsing–remitting (RR) disease course, and Expanded Disability Status Score (EDSS) less than or equal to 5.0 (Table 1). Candidate patients were required to have at least one medically documented clinical relapse within 12 months prior to randomization (for eligibility, a prestudy relapse was defined as neurologic signs and symptoms documented by review of the history with the subject or clearly documented in the medical record, of sufficient severity and duration to be determined by the investigator as consistent with an acute MS relapse; the relapse did not need to have been treated to qualify) and/or have progression of ≥1.0 points in EDSS in the previous year. All subjects had to have ≥ one Gd-enhancing brain lesion on a screening MRI, along with ≥2 T2 brain lesions consistent with MS on the baseline scan.
Table 1.
Baseline characteristics of enrolled patients.
| Characteristic | Interferon beta (n = 19) | MMF (n = 16) | p value* |
|---|---|---|---|
| Age – years, mean ± SD | 38.6 ± 9.4 | 37.4 ± 8.3 | 0.72 |
| Female – no (%) | 14 (73.7) | 11 (68.8) | 0.75 |
| Weight – kg, mean ± SD | 90.8 ± 21.3 | 82.9 ± 20.7 | 0.28 |
| Height – m, mean ± SD | 1.66 ± 0.10 | 1.71 ± 0.11 | 0.18 |
| Blood pressure – mmHg | |||
| Systolic blood pressure, mean ± SD | 124.4 ± 14.9 | 120.7 ± 13.9 | 0.46 |
| Diastolic blood pressure, mean ± SD | 79.7 ± 7.9 | 77.8 ± 12.5 | 0.60 |
| Race – no (%) | 0.24 | ||
| Caucasian | 11 (57.8) | 14 (87.5) | |
| African-American | 4 (21.1) | 1 (6.3) | |
| Other | 4 (21.1) | 1 (6.3) | |
| Disease duration in months, mean ± SD | 33.3 ± 46.4 | 30.9 ± 38.5 | 0.87 |
| Relapses within last 12 month prior to the study, no. (%) | 0.88 | ||
| 0 | 0 (0.0) | 1 (6.3) | |
| 1 | 16 (84.2) | 13 (81.3) | |
| 2 | 2 (10.5) | 2(12.5) | |
| 3 | 1 (5.3) | 0 (0.0) | |
| EDSS, mean ± SD, median (min – max) | 2.5 ± 1.3, 2.3 (0.0–6.0) | 2.6 ± 1.2, 2.3 (0.8–5.3) | 0.85 |
| MSFC | −0.12 ± 0.58 | 0.13 ± 0.94 | 0.37 |
| Arm, mean ± SD | −0.47 ± 1.35 | 0.24 ± 1.21 | 0.11 |
| Leg, mean ± SD | 0.04 ± 0.56 | −0.05 ± 1.36 | 0.81 |
| PASAT, mean ± SD | −0.36 ± 1.27 | 0.20 ± 0.98 | 0.16 |
| Quality of life (QOL-54) | |||
| Physical, mean ± SD | 58.2 ± 20.0 | 66.0 ± 16.6 | 0.22 |
| Emotional, mean ± SD | 58.3 ± 21.9 | 70.2 ± 19.6 | 0.10 |
| Beck’s Depression Index, mean ± SD | 13.2 ± 10.1 | 12.9 ± 9.9 | 0.93 |
| # No Gd lesions, mean ± SD, median (min – max) | 5.7 ± 7.1, 2 (1–24) | 4.3 ± 6.5, 2 (1–27) | 0.56 |
| # No T2 lesions, mean ± SD, median (min – max) | 34.8 ± 24.9, 28 (2–82) | 31.6 ± 18.3, 30 (4–65) | 0.82 |
| # Combined active lesions, mean ± SD, median (min – max) | 40.5 ± 27.9, 32(3–96) | 35.9 ± 19.6, 39.5(5–69) | 0.79 |
| T2-LV (ml), mean ± SD, median (min – max) | 11.6 ± 13.7, 7.2 (0.2–46.3) | 11.9 ± 7.1, 2.6 (1.3–57.2) | 0.94 |
| T1-LV (ml), mean ± SD, median (min – max) | 1.8 ± 4.7, 0.5 (0–20.8) | 1.2 ± 2, 0.3 (0–6.8) | 0.69 |
| Gd-LV (ml), mean ± SD, median (min – max) | 0.5 ± 1, 0.1 (1–4) | 0.6 ± 1, 0.2 (0.02–3.5) | 0.93 |
SD, standard deviation; number (no.); EDSS, Expanded Disability Status Scale; MSFC, Multiple Sclerosis Functional Composite; PASAT, Paced Auditory Serial Addition Tests; QOL, Quality of Life; LV, lesion volume.
Baseline characteristics were compared by either parametric or nonparametric methods where appropriate.
Exclusion criteria
Patients were excluded from participation if they did not fulfill all of the inclusion criteria and if they received treatment 3 months prior to study entry with any standard DMT (interferon-beta and glatiramer acetate, intravenous immune globulin (IVIG) or plasmapheresis). Further, patients were excluded if they previously received treatment 12 months prior to study entry with immunosuppressant agents such as mitoxantrone, cyclophosphamide, cladribine, fludarabine, cyclosporine, total body irradiation or any other immunomodulatory therapies (e.g., azathioprine, methotrexate, MMF, natalizumab, or other immunomodulators/monoclonal agents). Patients could not be enrolled if they received steroid treatment 30 days prior to the baseline MRI scan date. Also excluded were women who were pregnant, lactating or of childbearing age, who did not consent to utilize approved contraceptive use during the study.
Conduct of the study, randomization, and study discontinuation
This investigation maintained an MRI-blinded treatment assignment throughout the duration of the study. Further, blinded clinical study personnel included the certified EDSS examiner, Multiple Sclerosis Functional Composite (MSFC) technician, and ophthalmology technician (who completed all visual assessments). The patients and treating physicians were not blinded to treatment assignment. The reason for this was that we were unable to purchase identically appearing placebo Avonex injection kits, and as such were thereby precluded from conducting a ‘double-dummy’ controlled trial. An investigational drug services pharmacist created the randomization scheme and dispensed study medications to the nurse coordinator.
Following completion of the consent process, each patient underwent baseline assessments (physical exam by the treating neurologist, EKG, chest X-ray, and laboratory assessments for human immunodeficiency virus (HIV), rapid plasma reagin (RPR), and hepatitis B) for safety prior to initiation of study medication. Following run-in and qualifying MRI brain scans, patients had six additional monthly MRIs with Gd contrast performed at day 0, 60, 90, 120, 150 and 180, in this head-to-head comparison study. Neurological assessments included the Kurtzke EDSS and the MSFC (comprised of the 9-Hole Peg Test, Paced Auditory Serial Addition Test, 25-Foot Timed Walk and the Sloan Low-Contrast Letter Acuity Chart – 1.25%). These assessments were performed every 3 months following the baseline assessment for 180 days. Laboratory investigations (complete blood count, comprehensive metabolic panel and pregnancy test) were done at the initiation of study medication (day 0) and again at day 30, 60, 90 and 180. Questionnaires were administered by the nurse coordinator at baseline and day 180 and included the MS Quality of Life-54 (MSQOL-54), Modified Fatigue Impact Scale (MFIS)-21 and the Beck’s Depression Index (BDI)-21.
Randomization and administration of study medications
Patients were randomized by the study pharmacist to receive either Avonex or CellCept, and began treatment at day 0. Initiation of study medication did not exceed 60 days from the date of the qualifying MRI scan. Avonex was initiated at ¼ of the target dose for the first week, and then increased by ¼ dose weekly until the full dose was achieved (i.e. ¼ dose at week 1, ½ dose at week 2, ¾ dose at week 3, and full dose at week 4). CellCept was initiated at 500 mg twice daily taken on an empty stomach (defined by 1 h before or 2 h after a meal). This dose was continued for the first 2 weeks and then increased to 1000 mg twice daily thereafter. As such, treatment titration took place over 1 month as above, and then maintenance treatment was continued for five consecutive months. We recognized that a ‘standard’ target dose regimen for CellCept does not take into account potentially important pharmacologic factors such as body weight, plasma peak and trough drug levels, and IMD enzyme inhibition effects (perhaps signifying pharmacogenomic differences among patients, possibly related to IMD gene polymorphisms). At the time when our study was planned, we had no evidence-based literature, nor available assay systems from which to determine ‘appropriate’ and validated dosing schemes in individual patients. Similar limitations may also apply to interferon beta and other MS therapies.
Reasons for discontinuation
Conditions in which a patient could have been discontinued from the study included worsening of the patient’s condition (requiring more aggressive therapy outside of the trial), study medication complications and/or intolerable side effects, a patient’s voluntary withdrawal, the investigator’s decision (based on risk/benefit ratio for each individual patient), a patient’s repeated failure to comply with study procedures, inability to complete all research assessments, and/or closure of the study by either the sponsor or the FDA.
Safety evaluation
The objective of our safety surveillance plan was to compare MMF with interferon beta monotherapy groups with respect to patient-reported adverse events, and to assess for any laboratory derangements during the course of treatment.
Treatment of exacerbations
During each of the study visits, all patients were reminded to report any new symptoms and/or new concomitant medications (including over-the-counter supplements), to the nurse coordinator. Patients with suspected exacerbations were instructed to return to the clinic to be evaluated. If the treating neurologist decided that worsening of the clinical course was due to an MS exacerbation, the patient was then evaluated by the blinded EDSS examiner and MSFC technician. Patients were then offered treatment with either 1 g of methylprednisolone administered intravenously (or orally) daily for 3 days (or dexamethasone at 100 mg given i.v. or orally b.i.d. for 3 days) without a taper. If the acute exacerbation occurred within 30 days prior to the anticipated commencement of the therapeutic phase of the study, treatment with either interferon beta or MMF was delayed such that there was at least 30 days between receiving the last dose of steroid medication and the beginning of drug therapy, and also 60 days from the onset of the exacerbation. MRI assessments at different time points during the study were not delayed because of exacerbations or treatment with steroids. Baseline MRI was obtained prior to any steroid treatment.
Outcome measures
Measurement of inflammatory brain MRI indices represented the primary safety and exploratory efficacy outcome measures, and included a change in the cumulative mean number of new Gd-enhancing lesions, new T2 lesions, and combined active lesions (CAL). The secondary outcomes included clinical relapse and disability-based measures. Tertiary measures included two nonconventional MRI measures (magnetization-transfer imaging [MTI] and diffusion-tensor imaging [DTI]; the subject of a subsequent publication), MSFC, MSQOL-54, FIS and BDI.
MRI methods
MRI study protocol
All patients underwent serial MRI scans of the brain at screening (unless the patient had a qualifying MRI done at an outside facility within 30 days with at least one Gd-lesion), and at the following time points: days 0, 60, 90, 120, 150 and 180 (the nonconventional MRI measures were performed only at days 0, 90 and 180).
The MRI was performed at the Dallas site on a Philips 1.5T scanner, at the Buffalo site on a General Electric 1.5T scanner and at the Michigan site on a Siemens 1.5T scanner. The protocol was standardized by the central MRI reading center (Buffalo) and then implemented at all sites. We performed a dual spin echo (SE) sequence (PD/T2), 3D-spoiled gradient-recalled (SPGR) T1-weighed image (T1-WI), pre- and postcontrast SE T1-WI (the postcontrast sequence was obtained 5 min after administration of contrast agent as a single-dose, intravenous bolus [0.1 mMol/kg Gd-DTPA]), fast fluid attenuated inversion recovery (FLAIR), MTI and DTI sequences. The acquisition parameters are summarized in the supplemental data.
Supplemental data
The axial dual SE sequence was acquired with TE 12/95, TR 3000, NEX 1, ETL 14, FOV 25.6, matrix 256 × 192, 3-mm slice thickness with a total of 48 slices, no gap, and scan time 3 : 37 min. The axial 3D-SPGR T1-WI scan was acquired with FOV 24, matrix 256 × 192, 1.5-mm thickness, 110 slices, no gap, TE 6, TR 25, NEX 1, FLIP 30, scan time 9 : 11 min; axial SE T1-WI pre- and postcontrast with FOV 25.6, matrix 256 × 256, 48 slices, 3-mm thickness, no gap, TE 9, TR 600, NEX 1, scan time 4 : 07 min (the postcontrast sequence was obtained 5 min after administration of contrast agent as a single-dose intravenous bolus [0.1 mMol/kg Gd-DTPA]) and axial FLAIR with FOV 24 cm, matrix 256 × 192, 48 slices, 3-mm thickness, no gap, TE 120, TI 2100, TR 8500, ETL 22, NEX 1, with scan time of 3 : 25 min. The MTI was acquired with 3D-GE sequence (TR, 50 ms; NEX, 2; flip angle, 10°; matrix size, 256 × 160; FOV, 24 cm; 3-mm thickness; scan time: 4 : 36 min both with [MS] and without [M0] a pre-pulse [1500 Hz off-water resonance pulse]). DTI consisted of axial; TR, 9000 gated; matrix size, 64 × 64, FOV, 24 cm; 3 b ∼0 smm-2 images; 60 DW images with δ = 32 ms, Δ = 40 ms, and max b = 1000 smm-2; slices, 42; 3-mm thickness, directions, 11; scan time: 2 : 06 min.
MRI analysis
The central screening and on-site image analysis validation was performed at the Buffalo Neuroimaging Analysis Center, Department of Neurology, University at Buffalo, Buffalo, NY. The operators and neuroradiologists were blinded to the patients’ clinical characteristics, clinical status, and treatment assignment.
Lesion activity analysis
A new Gd-enhancing lesion was defined as a typical area of hyperintense signal on postcontrast T1-WI. We calculated the mean cumulative number of Gd-enhancing lesions per patient over the 180-day treatment period, defined as the period following the first month to the last month of this head-to-head comparison study (day 180). Other Gd-based MRI lesion activity outcomes included the number of total new Gd-enhancing lesions per patient and the number of persistent Gd-enhancing lesions (enhancing lesions that had also been present on the previous monthly scan).
A new or newly enlarging lesion on T2-weighted images was defined as a rounded or oval lesion arising from an area previously considered as normal appearing brain tissue (NABT) and/or showing an identifiable increase in size from a previously stable-appearing lesion. We measured the mean number of new T2 lesions per patient over the 180-day treatment period, defined as the period following the first month to the last month of the comparison study (day 180). We also determined the mean number of new CALs, as the number of new enhancing lesions plus the number of new or newly enlarging, nonenhancing lesions on T2-WI. An active scan was defined as showing any new, enlarging or recurrent lesion(s) on postcontrast T1- and T2-WI. The calculations of new Gd-enhancing, T2 lesions, CAL, and the proportion of active scans were based on semi-automated tracing methods applied to computer-displayed images at days 0, 60, 90, 120, 150 and 180.
Quantitative lesion analysis
The number and volume of Gd, T2 and T1 lesions were measured using a semi-automated edge detection contouring-thresholding technique, as previously described [Zivadinov et al. 2004, 2001].
Statistical analysis
Analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina). MRI endpoints were fitted with a negative binomial model accounting for overdispersion. Baseline and repeated measurements over time were also adjusted for the above-characterized end points, with the use of generalized estimating equations (GEE) in Genmod procedure. Mixed-effect models were applied for clinical end points adjusted by baseline as well. In the calculation of adverse event incidence rate, duration was the average in each treatment group from day 0 to day 180. Type I error for significance was set at the 0.05 level for all analyses.
Results
Patient demographic, clinical and MRI characteristics at baseline
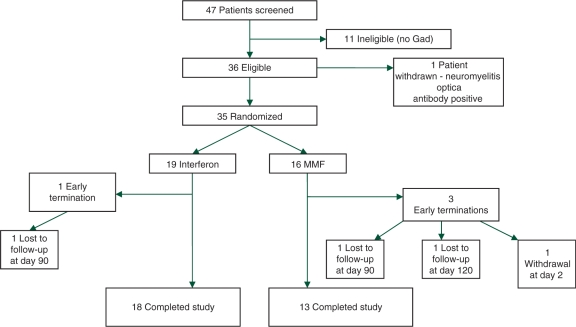
Of the 47 patients screened for the study, 35 patients were enrolled and randomized from three centers (Dallas, n = 23; Michigan, n = 9, and Buffalo, n = 3). The reasons for screening failures are outlined in Figure 1. The patient characteristics were well matched at baseline (Table 1) and not significantly different between the two parallel treatment groups. The mean age was 38.6 years in the interferon beta group and 37.4 years in the MMF arm, whereas the mean disease duration was 33.3 and 30.9 months respectively. The median EDSS in the interferon beta and MMF groups were 2.5 and 2.6. There were a higher proportion of African-Americans and patients with ‘other race’ (other than Caucasian) designation in the interferon beta compared with the MMF treatment arm, but this was not statistically significant. Both groups showed a high number of T2 lesions on the baseline scan (mean of 34.8 in the interferon beta group and 31.6 in the MMF arm), and showed high acute MRI activity at baseline (mean no. of Gd lesions of 5.7 in the interferon beta arm and 4.3 in the MMF arm).
Figure 1.
Enrollment of study patients, discontinuation and study completion.
Disposition and dropouts
Of the 35 patients enrolled in the study, 19 were randomized to interferon beta and 16 to MMF treatment (Figure 1). Four patients did not complete the study, but were entered into the intention-to-treat (ITT) analysis. Of the three dropout patients in the MMF arm, one patient voluntarily withdrew due to intolerable side effects (severe change in mood) at day 2 after receiving only two doses of MMF. The primary investigator classified the adverse event as ‘not likely’ related to the study medication. The other two early termination patients in the MMF treatment arm were lost to follow-up at day 120. One patient in the Avonex arm was lost to follow-up at day 120.
MRI outcomes
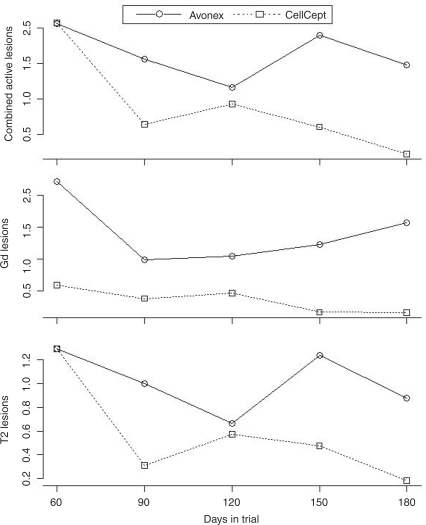
Least square means of active Gd, T2 and CAL lesions over time, were adjusted by each corresponding baseline measure at day 0, and is shown in Figure 2. There was no significant treatment effect observed for new T2 active lesions (p = 0.1), new active Gd lesions (p = 0.29) or for CAL (p = 0.15) between the two treatment arms.
Figure 2.
Least square means of combined active lesions, gadolinium (Gd)-enhancing lesions, and T2 lesions over time adjusted by respective baseline at day 0. There was no significant treatment effect observed for new T2 active lesions (p = 0.1), new active Gd lesions (p = 0.29), or for combined active lesions (CAL) (p = 0.15).
The mean cumulative number of Gd active lesions over the entire 180 days of the trial was 7.2 ± 17.4 for Interferon beta and 1.1 ± 1.8 for the MMF arm. During the 180 days of the study, 52.6% of the T1-postcontrast scans were active in the interferon beta arm versus only 40% of the scans in the MMF arm (p = NS). In total, ten patients in the Interferon beta arm developed a total of 137 new Gd-enhancing lesions over 180 days, while 7 patients in the MMF arm developed a total of only 17 new Gd-enhancing lesions (p = NS). The following represents the distribution of the Gd-enhancing lesions in the interferon beta arm: five patients had one lesion, one patient had six, one had 13, one had 19, one had 20, and one had 74 lesions. In the MMF arm: two patients had one lesion, one patient had two, one had three, one had four and one had six. The two treatment groups had one patient each, with a single persistent Gd-enhancing lesion. Similar trends in favor of MMF were identified on analyses of CAL and new T2 lesions, as shown in Figure 2. The mean cumulative number of new CAL over 180 days was 11.4 ± 24.9 for the interferon beta arm and 3 ± 2 for the MMF arm (p = NS), whereas the mean cumulative number of new T2 lesions over 180 days was 9.7 ± 25 for the interferon beta arm and 2.6 ± 1.4 for the MMF arm (p = NS).
Clinical outcomes
Five confirmed relapses occurred during the study, three in the interferon beta group (at 60 and 120 days) and two in the MMF group (at 60 and 90 days). All five attacks were categorically designated as mild by the evaluating neurologists. Of 19 patients in the interferon beta arm, 17 (89.5%) were relapse-free (one patient presented with two relapses) and of 16 patients in the MMF arm, 14 (87.5%) were relapse free (p = NS). No differences in other functional outcomes were observed between the two treatments arms at 90 and 180 days of the study (Table 2).
Table 2.
Functional outcomes by treatment assignment.
| Variable | Interferon beta (mean) | MMF (mean) | p value |
|---|---|---|---|
| EDSS | |||
| Day 90 | 2.05 | 1.74 | 0.45 |
| Day 180 | 2.03 | 1.76 | 0.51 |
| MSFC | |||
| Day 90 | −0.24 | 0.19 | 0.13 |
| Day 180 | −0.002 | 0.34 | 0.23 |
| MSFC – leg | |||
| Day 90 | −0.40 | −0.23 | 0.66 |
| Day 180 | −0.07 | −0.05 | 0.97 |
| MSFC – arm | |||
| Day 90 | 0.008 | 0.19 | 0.37 |
| Day 180 | 0.20 | 0.35 | 0.46 |
| MSFC – PASAT | |||
| Day 90 | −0.01 | 0.22 | 0.26 |
| Day 180 | 0.17 | 0.41 | 0.26 |
| Ambulation Index | |||
| Day 90 | 0.68 | 0.87 | 0.51 |
| Day 180 | 0.84 | 0.94 | 0.86 |
| Sloan visual acuity | |||
| Day 90 | 25.40 | 32.29 | 0.12 |
| Day 180 | 27.91 | 24.90 | 0.50 |
| Quality of life (QOL-54) – physical, day 180 | 63.07 | 67.59 | 0.32 |
| Quality of life (QOL-54) – emotional, day 180 | 61.31 | 66.26 | 0.37 |
| Fatigue Impact Scale - day 180 | 38 | 33.2 | 0.51 |
| Beck’s depression index - day 180 | 14.54 | 8.64 | 0.10 |
EDSS, Expanded Disability Status Scale, MSFC; Multiple Sclerosis Functional Composite; PASAT, Paced Auditory Serial Addition Tests; QOL, quality of life.
The differences were compared by either parametric or non-parametric method where appropriate.
Safety/tolerability
Both interferon beta and MMF appeared safe and well tolerated. The total number of AEs in the interferon beta group was not significantly different to those documented in the MMF group over the 180 days of the study (93 versus 71, p = NS) (Table 3). Overall, 13 (68.4%) patients in the interferon beta arm and eight (50%) in the MMF arm reported at least one AE over the study period (p = NS). There was a higher proportion of patients describing pain, weakness, dizziness, fatigue, numbness, flu-like symptoms, depression, nausea, sinusitis, anxiety, trauma, speech problems, hemorrhoids, site reactions, suicidal thoughts and trigeminal symptoms in the interferon beta arm, whereas headache, diarrhea, itching/pruritus, upper respiratory tract infection, tooth infection, eye infection, metal taste, influenza, ear infection, and bleeding of the nose occurred more frequently in the MMF arm (Table 3). Overall pain, urinary tract infections, headache, weakness, dizziness and diarrhea were the most common AEs reported.
Table 3.
Adverse events (AE) by treatment assignment.
| Adverse events, n (%) | Interferon beta (n = 19) | MMF (n = 16) |
|---|---|---|
| All/any AE | 93 | 71 |
| Pain | ||
| Extremities | 13 (68.4%) | 8 (50%) |
| Back pain | 6 (31.6%) | 2 (12.5%) |
| Abdominal pain | 1 (5.3%) | 3 (18.6%) |
| Facial | 3 (15.8%) | 4 (25%) |
| Urinary tract infection | 6 (31.6%) | 6 (37.5%) |
| Headache | 3 (15.8%) | 6 (37.5%) |
| Weakness | 6 (31.6%) | 2 (12.5%) |
| Dizziness | 5 (26.3%) | 0 |
| Diarrhea | 1 (5.3%) | 5 (31.3%) |
| Fatigue | 4 (21.1%) | 2 (12.5%) |
| Numbness | 4 (21.1%) | 2 (12.5%) |
| Itching/pruritis | 2 (10.5%) | 4 (25%) |
| Flu-like symptoms | 4 (21.1%) | 0 |
| Depression | 4 (21.1%) | 0 |
| Upper respiratory infection | 0 | 4 (25%) |
| MS relapse | 3 (15.8%) | 2 (12.5%) |
| Laboratory abnormalities | 3 (15.8%) | 3 (18.6%) |
| Nausea | 3 (15.8%) | 1 (6.3%) |
| Sinusitis | 3 (15.8%) | 0 |
| Tooth infection | 1 (5.3%) | 3 (18.6%) |
| Eye infection | 0 | 3 (18.6%) |
| Metal taste | 1 | 3 (18.6%) |
| Influenza | 2 (10.5%) | 3 (18.6%) |
| Anxiety | 2 (10.5%) | 1 (6.3%) |
| Trauma | 2 (10.5%) | 0 |
| Speech problems | 2 (10.5%) | 0 |
| Hemorrhoids | 2 (10.5%) | 0 |
| Site reaction | 2 (10.5%) | 0 |
| Insomnia | 1 (5.3%) | 1 (6.3%) |
| Abscess | 1 (5.3%) | 0 |
| Suicidal thoughts | 1 (5.3%) | 0 |
| Trigeminal symptoms | 1 (5.3%) | 0 |
| Incontinence | 1 (5.3%) | 1 (6.3%) |
| Bleeding of the nose | 0 | 1 (6.3%) |
| Ear infection | 0 | 1 (6.3%) |
The adverse events are listed in descending order of frequency. The differences in total number and frequency of adverse events between the two treatment arms are shown.
No differences in the severity of AEs occurred between the two treatment arms. There were no serious AEs in either treatment group (no malignancies, opportunistic infections or tuberculosis), except for the occurrence of a right thigh abscess in one patient in the interferon beta arm. Of all the AEs in the interferon beta group, 74.4% were deemed unlikely related to treatment, whereas of all AEs in the MMF arm, 65% were considered to be unlikely related to the treatment (p = NS). While not significant, we did identify a higher proportion of infections in the MMF treated patients. No differences were detected in the frequency of laboratory derangements between the two treatment arms (three in the interferon beta group and three in the MMF group) (Table 3).
Racial effects on study outcomes
Given the higher proportion of African-Americans and patients with ‘other race’ designations (other than Caucasian) in the interferon beta arm, we investigated the ‘race effect’ on study outcomes between the two different arms. No significant differences were found for any of the clinical, laboratory or radiographic analyses.
Discussion
From the patient’s quality-of-life perspective, the availability of effective, convenient and safe oral disease modification for MS would constitute a monumental advance. Since the FDA approval of the first DMT for MS in 1993 (Betaseron), the subsequent treatment options have all been parenterally administered agents (interferon beta1a, glatiramer acetate, mitoxantrone, and most recently natalizumab) [Frohman et al. 2006; Corboy et al. 2003; Goodin et al. 2002]. Both physicians and patients now expectantly look forward to the approval of oral formulations that appear to exert impressive efficacy on clinical and radiographic aspects of disease activity, in addition to potential neuroprotective effects, and superb tolerability and convenience.
An advantage of conventional MS DMT is that there have been no data linking interferon or glatiramer acetate treatment to the serious and potentially life-threatening opportunistic infections (OIs) that have been associated with the potent immunosuppressive agents (e.g. natalizumab, rituximab, azathioprine, MMF, cladribine, and fingolimod) [Berger and Houff, 2009; Cohen, 2009; Hemmer et al. 2006]. All of the newer agents have limited short-term safety data when compared with the interferons and glatiramer acetate. Further, mitoxantrone has been associated with the risk of potentially serious cardiotoxicity and the development of leukemias [Ellis and Boggild, 2009; Moses and Brandes, 2008; Goodin et al. 2003]. Nevertheless, the greater efficacy, improved convenience and adherence of the newer oral therapies will likely provide compelling justification for the application of these more expensive and higher risk approaches, in carefully selected patients.
There is an expanding literature suggesting that MMF has the ability to effectively treat a broad diversity of inflammatory disorders. These have included lupus [Karim et al. 2002; Lui et al. 2002; Schanz et al. 2002], cANCA vasculitis [Waiser et al. 1999], Takayasu’s arteritis [Daini et al. 1999], myasthenia gravis [Chaudry et al. 2001; Ciafaloni et al. 2001; Mowzoon et al. 2001; Hauser et al. 1998; Schneizer et al. 2001], chronic inflammatory demyelinating polyneuropathy [Chaudry et al. 2001; Mowzoon et al. 2001;], polymyositis [Schneider et al. 2002; Chaudry et al. 2001; Mowzoon et al. 2001], treatment of refractory skin manifestations of dermatomyositis [Gelber et al. 2000], inclusion body myositis [Mowzoon et al. 2001], psoriasis [Schrader et al. 2002; Ameen et al. 2001], neuromyelitis optica (NMO) [Schrader et al. 2002; Ameen et al. 2001], and Susac’s disease [Pawate et al. 2009]. A small open-label surveillance study involving seven MS patients treated with MMF was reported in 2001 and suggested evidence of tolerability and potential efficacy in this small cohort [Ahrens et al. 2001]. We extended this observation with our open-label surveillance study of MMF (CellCept) in 79 patients with MS [Frohman et al. 2004]. Vermersch and colleagues conducted an open-label pilot safety study in 30 MS patients treated with weekly intramuscular interferon beta 1a for at least 6 months, and who had at least one exacerbation within the last 6 months from the time when MMF was added [Vermersch et al. 2007]. Over 6 months of systematic assessment, there appeared to be both clinical and radiographic benefits demonstrated with acceptable safety. In a retrospective study involving 42 MS patients who were treated with either mitoxantrone or MMF, all appeared to either stabilize or exhibit improvement [Vukusic et al. 2004].
With the recent approval of generic status for MMF, we believe that the results presented in this study are potentially important to our MS patients, and for those of us who struggle to adequately and economically manage this complex and highly heterogeneous inflammatory disorder. If MMF can be confirmed as an effective DMT for MS in adequately powered controlled efficacy trials, both cost and our extensive experience with this agent in transplant medicine would make it an attractive oral option that could rival agents under development that will no doubt be financially exorbitant.
Our trial patients were representative of RRMS patients studied in previous clinical trials. Further, we specifically directed our treatment to those individuals with evidence of new inflammatory MRI activity, in order to assess safety of MMF when compared with a standard, effective, generally well-tolerated, and commonly utilized MS DMT, interferon beta. Over the course of the current 6 month pilot trial both agents were well tolerated in the majority of patients, validating the outcomes of our previous safety surveillance study in 79 MMF-treated patients [Frohman et al. 2004].
MRI metrics of MS disease activity represented the primary safety and efficacy outcome of our study. It should be emphasized that a potential confounding variable that could have influenced the outcomes we have characterized is the differential delay in drug action between MMF and interferon beta. We titrated to the target dose over 4 weeks with both agents. However, the onset effects may be quite different. As such, longer controlled trials along with measures of bioavailability (e.g. MxA levels after interferon injection), serum drug peak and trough concentration, and enzyme inhibition effects (for MMF) will be important features to consider in any class I randomized controlled comparison trials for efficacy and safety. While we did not identify any differences between our two study groups in terms of prestudy characteristics, in a small pilot study we cannot preclude the possibility that the retrospective assessment of relapses and corresponding treatment with steroids may represent a potential confounder.
We confirmed our principal hypothesis that MMF appeared safe compared with interferon beta, when considering any radiographic, clinical or laboratory measure. While not statistically significant, we observed that the mean cumulative number of CAL, Gd- and T2-active lesions over the entire 180 days of the trial was less in those treated with MMF versus interferon beta. During the 6 months of the study, 52.6% of the T1-postcontrast scans were active in the interferon beta arm, whereas only 40% of the scans were active in the MMF arm. Those patients in the interferon beta arm exhibited 137 new Gd-enhancing lesions, while 17 new Gd lesions were documented in seven patients treated with MMF (p = NS). Another observed trend (not significant) from our analyses was the distribution of new lesions per patient, which was higher in interferon beta compared to MMF-treated patients. Assessment of the number of CAL and new T2 lesions demonstrated similar trends. Clinical exacerbations were similar between the two groups, as was the categorical designation of attack severity (all were scored as mild).
Our study has a number of important limitations that must be characterized in order to put our observations into appropriate perspective. Within the scope of a pilot trial, we studied a population of relapsing MS patients who had brain MRI evidence of Gd-enhancing lesions in order to reduce the necessary sample size to adequately assess radiologic safety of MMF when compared with interferon beta, an established safe and effective therapy. Without doubt, the small number of enrolled patients thereby precludes us from generating any valid generalizations with respect to efficacy. Notwithstanding this limitation, some favorable trends were observed for MMF when compared with interferon beta. As with many early-stage exploratory and safety trials, any and all of the effects exerted on MRI indices of CNS inflammation and gliosis, must be corroborated in larger controlled trials. Similarly, there is still to date no evidence-based controlled studies demonstrating disease-modifying efficacy of MMF in MS.
Objective imaging metrics were utilized as the primary outcomes in our study. These measures are important as they provide structural and pathophysiological information regarding a treatment’s possible efficacy in controlling aspects of inflammation in MS that are detectable on scans, and that are estimated to occur at a rate that far exceeds the occurrence of clinical events. However, while there is an abundance of evidence that MRI is an effective biomarker for disease activity in MS, demonstration of a rigorously quantifiable linkage between new lesions, their accumulation, and microstructural alterations (demyelination, axonal transection, gliosis, etc.) to relevant clinical measures remains elusive.
With the advent of more sensitive and nonconventional imaging technology, we can begin to better characterize MS brain and spinal cord lesions, than has been feasible with traditional T2- and T1-weighted measures. Such capabilities will be germane to understanding the basis of the clinical–radiological paradox, which signifies the discrepancy between these older and nonspecific imaging markers and corresponding measures of clinical disability [Barkhof et al. 2009; Filippi and Rocca, 2009; Bar-Zohar et al. 2008].
While the primary outcome of our study utilized MRI metric-based analyses and involved blinded technicians and neuroradiologists, another limitation of our study is that the study patients and the treating physicians were informed of the randomized treatment assignment. We originally intended to perform a ‘double-dummy’ trial, but were unable to acquire identically appearing Avonex injection kits.
The expectation of type and intensity of treatment regimen can bias and confound any clinical investigation. However, unlike unblinded studies which seek to evaluate the differential efficacy of higher versus lower dose frequency of a particular therapy (which can influence those who believe that ‘more is better’; the ‘supersize effect’), when consenting study participants in our pilot investigation, we were clear to underscore the principle that both interferon beta and MMF both exert potent immunological effects on immune function that would appear to be fundamental to controlling our primary outcome measures, the development of new MRI lesions. Further, we informed patients of the long-term efficacy and safety data associated with interferon beta therapy, but likewise discussed the long-standing utilization of MMF in transplant recipients, and in patients with a variety of immune-mediated disorders. We informed patients that MMF’s action to inhibit immune rejection in those with organ transplants, is analogous to its potential action in reducing inflammation and tissue damage in autoimmune diseases (a variation of rejection), including MS. Despite this education, interferon beta is an FDA approved and established DMT for MS, while MMF is not. As such, this might have biased the study in favor of interferon beta based on the possible expectation of either (1) receiving established therapy with interferon beta or (2) receiving unproven experimental treatment in the case of MMF. Alternately, we could have anticipated that parenteral therapy with interferon beta might have been associated with a lower rate of adherence when compared with oral MMF, potentially impacting upon efficacy. The reciprocal consideration might also be true, that weekly intramuscular interferon beta adherence might have been anticipated to be superior to twice-daily MMF, which requires administration on an empty stomach. Notwithstanding such potential confounders of a non-double-dummy trial, and the potential bias that could result from the knowledge of treatment assignment, adherence rates were indistinguishable between the two groups.
Given the similar risk of producing serious OIs that has been documented with other immunosuppressive agents, we anticipate that MMF, if successful in demonstrating safety and efficacy in larger studies, will likely be considered a more intensive and higher risk treatment strategy when compared with the parenterally administered interferons and glatiramer acetate. For instance, a number of safety concerns have been associated with the use of MMF, the most ominous of which has been the confirmation of progressive multifocal leukoencephalopathy (PML) [Food and Drug Administration 2009]. Most of the MMF-associated PML cases have occurred in organ transplant recipients, and those with complicated systemic lupus erythematosis.
Despite the limitations of our small pilot study, we believe that our data provide preliminary evidence for the safety and tolerability of MMF in comparison with an established, effective, safe, and generally well-tolerated parenteral MS therapy. Ultimately these results must be corroborated in larger controlled trials to determine if this convenient, oral, and now cost-effective immunosuppressant agent is to be confirmed as a safe and effective candidate agent for inclusion into the MS treatment armamentarium.
Conflict of interest statement
This investigator-initiated study (EMF, RZ, and GC) was funded by an unrestricted grant from Aspreva Pharmaceuticals. This entity played no role whatsoever in the design, execution or analysis of this trial. Furthermore, the sponsor had no role in the drafting or editing of the manuscript. We (EMF, RZ, GC) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Ahrens N., Salama A., Haas J. (2001) Mycophenolate-mofetil in the treatment of refractory multiple sclerosis. J Neurol 248: 713–714 [DOI] [PubMed] [Google Scholar]
- Allison A.C., Eugui E.M. (1993) The design and development of an immunosuppressive drug, mycophenolate mofetil. Springer Semin Immunopathol 14: 353–380 [DOI] [PubMed] [Google Scholar]
- Allison A.C., Kowalski W.J., Muller C.D., Eugui E.M. (1993) Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci 696: 63–87 [DOI] [PubMed] [Google Scholar]
- Ameen M., Smith H.R., Barker J.N. (2001) Combined mycophenolate mofetil and cyclosporin therapy for severe recalcitrant psoriasis. Clin Exp Dermatol 26: 480–483 [DOI] [PubMed] [Google Scholar]
- Bar-Zohar D., Agosta F., Goldstaub D., Filippi M. (2008) Magnetic resonance imaging metrics and their correlation with clinical outcomes in multiple sclerosis: a review of the literature and future perspectives. Mult Scler 14: 719–727 [DOI] [PubMed] [Google Scholar]
- Barkhof F., Filippi M. (2009) MRI – the perfect surrogate marker for multiple sclerosis? Nat Rev Neurol 5: 182–183 [DOI] [PubMed] [Google Scholar]
- Barten M.J., van Gelder T., Gummert J.F., Boeke K., Shorthouse R., Billingham M.E., et al. (2002a) Pharmacodynamics of mycophenolate mofetil after heart transplantation: new mechanisms of action and correlations with histologic severity of graft rejection. Am J Transplant 2: 719–732 [DOI] [PubMed] [Google Scholar]
- Barten M.J., van Gelder T., Gummert J.F., Shorthouse R., Morris R.E. (2002b) Novel assays of multiple lymphocyte functions in whole blood measure: new mechanisms of action of mycophenolate mofetil in vivo. Transpl Immunol 10: 1–14 [DOI] [PubMed] [Google Scholar]
- Berger J.R., Houff S. (2009) Opportunistic infections and other risks with newer multiple sclerosis therapies. Ann Neurol 65: 367–377 [DOI] [PubMed] [Google Scholar]
- Blaheta R.A., Leckel K., Wittig B., Zenker D., Oppermann E., Harder S., et al. (1999) Mycophenolate mofetil impairs transendothelial migration of allogeneic CD4 and CD8 T-cells. Transplant Proc 31: 1250–1252 [DOI] [PubMed] [Google Scholar]
- Chaudhry V., Cornblath D.R., Griffin J.W., O’Brien R., Drachman D.B. (2001) Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology 56: 94–96 [DOI] [PubMed] [Google Scholar]
- Ciafaloni E., Massey J.M., Tucker-Lipscomb B., Sanders D.B. (2001) Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology 56: 97–99 [DOI] [PubMed] [Google Scholar]
- Cohen J.A. (2009) Emerging therapies for relapsing multiple sclerosis. Arch Neurol 66: 821–828 [DOI] [PubMed] [Google Scholar]
- Corboy J.R., Goodin D.S., Frohman E.M. (2003) Disease-modifying therapies for multiple sclerosis. Curr Treat Options Neurol 5: 35–54 [DOI] [PubMed] [Google Scholar]
- Daina E., Schieppati A., Remuzzi G. (1999) Mycophenolate mofetil for the treatment of Takayasu arteritis: report of three cases. Ann Intern Med 130: 422–426 [DOI] [PubMed] [Google Scholar]
- Ellis R., Boggild M. (2009) Therapy-related acute leukaemia with Mitoxantrone: what is the risk and can we minimise it? Mult Scler 15: 505–508 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A. (2009) Functional MR imaging in multiple sclerosis. Neuroimaging Clin N Am 19: 59–70 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2009) Drug Safety Information for Health Care Professionals [online]. Available at: http://fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm072438.htm
- Frohman E.M., Brannon K., Racke M.K., Hawker K. (2004) Mycophenolate mofetil in multiple sclerosis. Clin Neuropharmacol 27: 80–83 [DOI] [PubMed] [Google Scholar]
- Frohman E.M., Racke M.K., Raine C.S. (2006) Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med 354: 942–955 [DOI] [PubMed] [Google Scholar]
- Gelber A.C., Nousari H.C., Wigley F.M. (2000) Mycophenolate mofetil in the treatment of severe skin manifestations of dermatomyositis: a series of 4 cases. J Rheumatol 27: 1542–1545 [PubMed] [Google Scholar]
- Goodin D.S., Arnason B.G., Coyle P.K., Frohman E.M., Paty D.W. (2003) The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 61: 1332–1338 [DOI] [PubMed] [Google Scholar]
- Goodin D.S., Frohman E.M., Garmany Jr G.P., Halper J., Likosky W.H., Lublin F.D., et al. (2002) Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 58: 169–178 [DOI] [PubMed] [Google Scholar]
- Hauser R.A., Malek A.R., Rosen R. (1998) Successful treatment of a patient with severe refractory myasthenia gravis using mycophenolate mofetil. Neurology 51: 912–913 [DOI] [PubMed] [Google Scholar]
- Hemmer B., Frohman E., Hartung H.P., Stuve O. (2006) Central nervous system infections - a potential complication of systemic immunotherapy. Curr Opin Neurol 19: 271–276 [DOI] [PubMed] [Google Scholar]
- Ishida H., Tanabe K., Furusawa M., Ishizuka T., Shimmura H., Tokumoto T., et al. (2002) Mycophenolate mofetil suppresses the production of anti-blood type anitbodies after renal transplantation across the abo blood barrier: ELISA to detect humoral activity. Transplantation 74: 1187–1189 [DOI] [PubMed] [Google Scholar]
- Jonsson C.A., Carlsten H. (2002) Mycophenolic acid inhibits inosine 5′-monophosphate dehydrogenase and suppresses production of pro-inflammatory cytokines, nitric oxide, and LDH in macrophages. Cell Immunol 216: 93–101 [DOI] [PubMed] [Google Scholar]
- Karim M.Y., Alba P., Cuadrado M.J., Abbs I.C., D’Cruz D.P., Khamashta M.A., et al. (2002) Mycophenolate mofetil for systemic lupus erythematosus refractory to other immunosuppressive agents. Rheumatology (Oxford) 8: 876–882 [DOI] [PubMed] [Google Scholar]
- Lui S.L., Tsang R., Wong D., Chan K.W., Chan T.M., Fung P.C., et al. (2002) Effect of mycophenolate mofetil on severity of nephritis and nitric oxide production in lupus-prone MRL/lpr mice. Lupus 11: 411–418 [DOI] [PubMed] [Google Scholar]
- Menge T., Weber M.S., Hemmer B., Kieseier B.C., von Büdingen H.C., Warnke C., et al. (2008) Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs 68: 2445–2468 [DOI] [PubMed] [Google Scholar]
- Miljkovic D., Samardzic T., Drakulic D., Stosic-Grujicic S., Trajkovic V. (2002) Immunosuppressants leflunomide and mycophenolic acid inhibit fibroblast IL-6 production by distinct mechanisms. Cytokine 19: 181–186 [DOI] [PubMed] [Google Scholar]
- Moses Jr H., Brandes D.W. (2008) Managing adverse effects of disease-modifying agents used for treatment of multiple sclerosis. Curr Med Res Opin 24: 2679–2690 [DOI] [PubMed] [Google Scholar]
- Mowzoon N., Sussman A., Bradley W.G. (2001) Mycophenolate (CellCept) treatment of myasthenia gravis, chronic inflammatory polyneuropathy and inclusion body myositis. J Neurol Sci 185: 119–122 [DOI] [PubMed] [Google Scholar]
- Noseworthy J.H., Lucchinetti C., Rodriguez M., Weinshenker B.G. (2000) Multiple sclerosis. N Engl J Med 343: 938–952 [DOI] [PubMed] [Google Scholar]
- O’Connor P.W., Li D., Freedman M.S., Bar-Or A., Rice G.P., Confavreux C., et al. (2006) A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66: 894–900 [DOI] [PubMed] [Google Scholar]
- Pawate S., Agarwal A., Moses H., Sriram S. (2009) The spectrum of Susac’s syndrome. Neurol Sci 30: 59–64 [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Edan G., Filippi M., Hartung H.P., Kappos L., et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 58: 840–846 [DOI] [PubMed] [Google Scholar]
- Schanz S., Ulmer A., Rassner G., Fierlbeck G. (2002) Successful treatment of subacute cutaneous lupus erythematosus with mycophenolate mofetil. Br J Dermatol 147: 174–178 [DOI] [PubMed] [Google Scholar]
- Schrader P., Mooser G., Peter R.U., Puhl W. (2002) [Preliminary results in the therapy of psoriatic arthritis with mycophenolate mofetil]. Z Rheumatol 61: 545–550 [DOI] [PubMed] [Google Scholar]
- Schneider C., Gold R., Reiners K., Toyka K.V. (2001) Mycophenolate mofetil in the therapy of severe myasthenia gravis. Eur Neurol 46: 79–82 [DOI] [PubMed] [Google Scholar]
- Schneider C., Gold R., Schafers M., Toyka K.V. (2002) Mycophenolate mofetil in the therapy of polymyositis associated with a polyautoimmune syndrome. Muscle Nerve 25: 286–288 [DOI] [PubMed] [Google Scholar]
- Treadaway K., Cutter G., Salter A., Lynch S., Simsarian J., Corboy J., et al. (2009) Factors that influence adherence with disease-modifying therapy in MS. J Neurol 256: 568–576 [DOI] [PubMed] [Google Scholar]
- Vermersch P., Waucquier N., Michelin E., Bourteel H., Stojkovic T., Ferriby D., et al. (2007) Combination of IFN beta-1a (Avonex) and mycophenolate mofetil (CellCept) in multiple sclerosis. Eur J Neurol 14: 85–89 [DOI] [PubMed] [Google Scholar]
- Vukusic S., Ducray F., Gignoux L. (2004) Mycophenolatemofetil: an open-label study in 42 MS patients. Neurology 62: 491–49114872041 [Google Scholar]
- Waiser J., Budde K., Braasch E., Neumayer H.H. (1999) Treatment of acute c-ANCA-positive vasculitis with mycophenolate mofetil. Am J Kidney Dis 34: e9–e9 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Bagnato F., Nasuelli D., Bastianello S., Bratina A., Locatelli L., et al. (2004) Short-term brain atrophy changes in relapsing-remitting multiple sclerosis. J Neurol Sci 223: 185–193 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Rudick R.A., De Masi R., Nasuelli D., Ukmar M., Pozzi-Mucelli R.S., et al. (2001) Effects of IV methylprednisolone on brain atrophy in relapsing-remitting MS. Neurology 57: 1239–1247 [DOI] [PubMed] [Google Scholar]