Tab. 3.
Predicted affinity of new escitalopram derivatives to SERT active site. The residual values (the biological activity for novel escitalopram derivatives differences to the parent biological activity) are in brackets.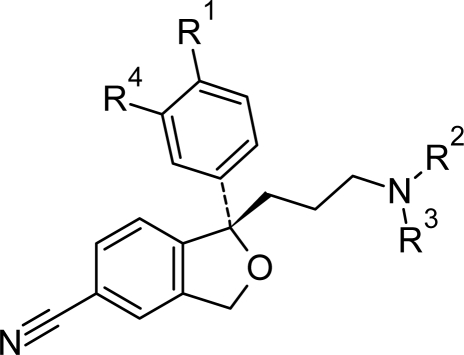
| Escitalopram derivative | Substituents | pKia | pKib | pKic | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||||
| Derivative 1 | Cl | CH3 | CH3 | H | 9.05(0.1) | 9.02(0.07) | 9.03(0.08) |
| Derivative 2 | Br | CH3 | CH3 | H | 9.04(0.09) | 9.01(0.06) | 9.03(0.08) |
| Derivative 3 | OH | CH3 | CH3 | H | 8.95(0) | 8.96(0.01) | 8.94(−0.01) |
| Derivative 4 | CH3 | CH3 | CH3 | H | 8.99(0.04) | 8.98(0.03) | 8.97(0.02) |
| Derivative 5 | NH-CH=O | CH3 | CH3 | H | 8.72(−0.23) | 8.76(−0.19) | 8.71(−0.24) |
| Derivative 6 | NO2 | CH3 | CH3 | H | 9.05(0.1) | 9.04(0.09) | 9.04(0.09) |
| Derivative 7 | OCH3 | CH3 | CH3 | H | 9.02(0.07) | 9.06(0.11) | 9.01(0.06) |
| Derivative 8 | F | CH3 | CH3 | F | 9.09(0.14) | 9.18(0.23) | 9.08(0.13) |
| Derivative 9 | F | CH3 | CH3 | Cl | 9.08(0.13) | 9.14(0.19) | 9.07(0.12) |
| Derivative 10 | F | CH3 | CH3 | allyl | 8.94(−0.01) | 8.95(0) | 8.93(−0.02) |
| Derivative 11 | F | CH3 | CH3 | ethyl | 9.16(0.21) | 9.16(0.21) | 9.15(0.2) |
| Derivative 12 | F | CH3 | CH3 | i-propyl | 8.91(−0.04) | 8.87(−0.08) | 8.9(−0.05) |
| Derivative 13 | F | CH3 | CH3 | OCH3 | 9.12(0.17) | 9.17(0.22) | 9.11(0.16) |
| Derivative 14 | F | CH3 | CH3 | t-butyl | 9.06(0.11) | 9.04(0.09) | 9.05(0.1) |
| Derivative 15 | F | CH3 | CH3 | OH | 8.95(0) | 9.03(0.08) | 8.94(−0.01) |
| Derivative 16 | F | H | H | H | 8.75(−0.2) | 8.68(−0.27) | 8.73(−0.22) |
| Derivative 17 | F | H | CH3 | H | 8.99(0.04) | 8.94(−0.01) | 8.98(0.03) |
| Derivative 18 | F | allyl | CH3 | H | 8.89(−0.06) | 8.86(−0.09) | 8.88(−0.07) |
| Derivative 19 | F | i-propyl | CH3 | H | 8.98(0.03) | 8.94(−0.01) | 8.96(0.01) |
| Derivative 20 | F | ethyl | ethyl | H | 9.38(0.43) | 9.35(0.4) | 9.37(0.42) |
| Derivative 21 | F | propyl | propyl | H | 9.36(0.41) | 9.28(0.33) | 9.34(0.39) |
| Derivative 22 | F | t-butyl | t-butyl | H | 9.23(0.28) | 9.14(0.19) | 9.22(0.27) |
| Derivative23 | F | ethyl | ethyl | F | 9.43(0.48) | 9.45(0.5) | 9.41(0.46) |
| Derivative24 | F | t-butyl | t-butyl | F | 9.32(0.37) | 9.31(0.36) | 9.31(0.36) |
Na-OH-phenyl atom probes;
K-OH-phenyl atom probes;
Ca-OH-phenyl atom probes.
