Abstract
Aims: The aim of this study was to probe the relationship between the subjective effects of alcohol and impulsive behavior in social drinkers. Methods: Fifty social drinkers performed a response-inhibition task before consuming alcohol. A 0.8-g/kg dose of alcohol was administered in a binge-like fashion (0.2 g/kg every 30 min) to the participants over a 2-h time period. Participants then completed questionnaires measuring stimulation, sedation and mood following consumption of alcohol. Linear regression analyses were performed by examining the relationship between performance on the response inhibition impulsivity task and subjective responses to alcohol (i.e. stimulation, sedation and arousal). Results: There was a significant positive relationship found between impulsive responding and self-reported sedation following alcohol consumption. Additionally, there was a significant negative relationship between behavioral impulsivity and self-reported stimulation and arousal following alcohol consumption. Conclusion: These results suggest that higher levels of impulsivity are associated with experiencing greater sedating than stimulating effects of alcohol. Individuals with high levels of impulsivity may be less sensitive to the stimulating effects of a specified dose of alcohol, which could lead to these individuals consuming more alcohol to experience the stimulating effects of alcohol.
INTRODUCTION
As alcohol abuse continues to cause economic, social and health problems for our society, identifying risk factors for alcohol problems becomes increasingly important. One such risk factor is differential sensitivity to the stimulating and sedating effects of alcohol. Previous studies have shown that subjective measurements of stimulation and sedation following alcohol consumption may predict risk of developing alcohol problems (Holdstock et al., 2000; Schuckit, 1994). Additionally, numerous studies have examined subjective responses to alcohol in populations that are genetically at risk for developing alcohol problems i.e. family history positive (FHP). These studies have found that FHP individuals report greater stimulation following alcohol consumption when compared with family history negative individuals (Erblich and Earleywine, 2003; King et al., 1997; Morzorati et al., 2002; Ramchandani et al., 1999). More specifically, individuals at risk for alcoholism experience greater stimulation-like effects during the ascending limb of the breath alcohol curve and attenuated sedative-like effects on the descending limb compared with those not at risk (Newlin and Thomson, 1990). In studies comparing heavy versus light drinkers, heavy drinkers experienced greater stimulant-like subjective effects and less sedative-like effects (Holdstock et al., 2000; King et al., 2002). The positive reinforcing effects of alcohol have been attributed to the stimulating effects (Earleywine, 1994; Peterson et al., 1996), so increased sensitivity to the stimulating effects may place an individual at greater risk for developing alcohol problems.
Another factor associated with increased risk for alcohol-related problems is impulsivity (Brook et al., 1995; Poikolainen, 2000; Simons et al., 2004; Verdejo-Garcia et al., 2007). Not only does acute alcohol administration increase impulsive behavior (Dougherty et al., 2008; Fillmore and Vogel-Sprott, 1999; Marczinski et al., 2005; Mulvihill et al., 1997; Ortner et al., 2003; Reynolds et al., 2006; Richards et al., 1999), but alcohol abusers report higher levels of trait impulsivity (Bjork et al., 2004; Mitchell et al., 2005; Whiteside and Lynam, 2003). Impulsivity is a multifaceted construct and many behaviors have been proposed to fall under the umbrella term impulsivity. Of the multiple components that have been proposed to underlie impulsivity, behavioral inhibition difficulty serves as a general vulnerability factor to substance-abuse problems (Verdejo-Garcia et al., 2008). Behavioral inhibition refers to the ability to inhibit a prepotent response, which becomes necessary when the prepotent response is inappropriate. Response-inhibition tasks are commonly used to assess deficits in behavioral inhibition, and alcohol-dependent individuals exhibit impaired performance on these tasks when compared with controls (Bjork et al., 2004; Kamarajan et al., 2005; Lawrence et al., 2009; Noel et al., 2007). Additionally, individuals who perform more impulsively on a response-inhibition task consume more alcohol during an ad lib procedure (Weafer and Fillmore, 2008), further strengthening the link between the behavioral inhibition component of impulsive behavior and alcohol-related problems.
While previous research has supported both subjective response to alcohol and impulsive behaviors as potential risk factors for alcohol misuse, the causal relationship between these two domains is not well understood. As part of a post hoc analysis within a population of social drinkers, a positive relationship between trait impulsivity and an increased desire to drink more alcohol following an alcohol prime was found (Rose and Grunsell, 2008). While many factors could contribute to this relationship, individuals with higher impulsivity in particular may be more sensitive to the stimulating/positive effects of the alcohol prime. This sensitivity, in turn, may then contribute to an increased desire to drink. A study conducted in college students found that lower levels of behavioral control (e.g. sensation seeking) was significantly correlated with ascending limb stimulation following a 0.85-g/kg alcohol dose (Erblich and Earleywine, 2003). While this study did not look directly at impulsivity and subjective response to alcohol, sensation seeking is a personality trait that is proposed to contribute to impulsivity (Verdejo-Garcia et al., 2010). To the best of our knowledge, there has not been a study that a priori aimed to examine the relationship between the behavioral inhibition component of impulsive behavior and sensitivity to the subjective effects of alcohol. If individuals who engage in impulsive behavior and individuals sensitive to the stimulating effects of alcohol are at higher risk for developing alcohol problems, one might expect impulsive individuals to endorse more stimulation and positive mood following alcohol administration.
The aim of this study was to test the directional relationship between impulsive behavior and subjective responses to alcohol in a population of social drinkers. An alcohol dose of 0.8 g/kg was administered in a binge-like manner according to the NIAAA definition of a binge (i.e. four 0.2-g/kg drinks over a 2-h time period; NIAAA, 2004). Impulsive behavior was measured using a response-inhibition task (GoStop Impulsivity paradigm; Dougherty et al., 2009) that has been shown to be sensitive to the acute effects of alcohol administration in social drinkers (Dougherty et al., 2008). Within this task, there are four stop delays (50, 150, 250 and 350 ms) used to discriminate impulsive responding, with the 150-ms stop delay providing the best discrimination between high- and low-impulsive individuals (Marsh et al., 2002). Subjective responses to a 0.8-g/kg alcohol dose were assessed using the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) and the profile of mood states (POMS; McNair et al., 1971). The study tested the hypothesis that individuals who exhibited higher levels of impulsive behavior (less behavioral inhibition) would report greater positive mood effects and more stimulant-like effects following alcohol consumption compared with low-impulsive individuals.
MATERIALS AND METHODS
Participants
Fifty healthy men (n = 34) and women (n = 16) were recruited from the community via television advertisements for a research study examining the behavioral effects of alcohol. A brief interview was first conducted over the telephone to assess medical history, illicit drug use and current drinking habits. Potential participants were invited to the laboratory for a screening visit. During the screening visit, a research assistant administered the modified Structured Clinical Interview for DSM-IV Disorders (First et al., 2002), the Wechsler Abbreviated Scale for Intelligence (Wechsler, 1999) and the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001); Additionally, a urine sample was collected to test for the presence of illicit drugs (Multi-drug 6 line urine screen; Innovacon, Inc., San Diego, CA, USA) and, in females for pregnancy (QuickVue; Quidel, San Diego, CA, USA).
Participants were included in the study if they had an AUDIT score ≤12, did not meet criteria for any Axis-I disorder within the last 6 months, had no psychoactive medication use within the last 6 months, a body mass index of less than 30 and reported at least one binge episode in the past 3 months. An AUDIT cutoff of ≤12 was chosen to exclude problematic or dependent drinkers (Conigrave et al., 1995). A binge episode was classified as four drinks (females) or five drinks (males) consumed within a 2-h period (NIAAA, 2004). This criterion ensured that all participants had current experience with the dose and timing of alcohol administration utilized in this study.
Participants provided written consent prior to entering the study and all procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
Study design
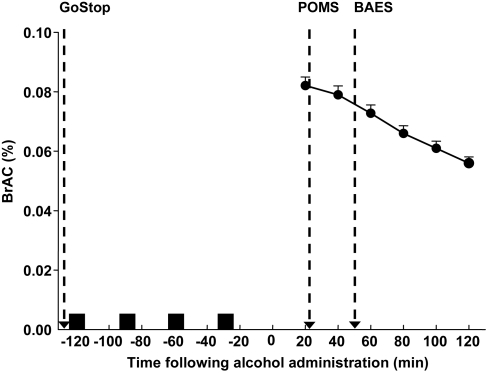
Participants who met study inclusion criteria reported to the laboratory for a second visit during which alcohol was administered. Upon arrival, a urine sample was collected and tested for the presence of illicit drugs and, in females, for pregnancy. Additionally, an expired air sample was collected and tested for the presence of alcohol (Intoxilyzer SD-5; CMI Inc., Owensboro, KY, USA). Participants first completed the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) and performed the GoStop Impulsivity paradigm (Dougherty et al., 2009). Alcohol administration commenced immediately following completion of the GoStop task (Fig. 1). Following completion of the alcohol administration procedure, the participants were administered the BAES ( Martin et al., 1993) and POMS (McNair et al., 1971) to assess subjective feelings related to the pharmacological effects of alcohol (Fig. 1).
Fig. 1.
Timeline of drink administration in relation to the measurements of impulsivity and subjective effects. Also shown is the mean (± SEM) BrAC curve following 0.8-g/kg 95% alcohol administered in a binge-like fashion (four drinks over 2 h). Arrows indicate times the GoStop Impulsivity Paradigm, the POMS and the BAES were completed by the participants. Black squares indicate times of alcohol drink administration.
Alcohol administration
Alcohol was administered in a manner to simulate an alcohol binge according to the guidelines set forth by NIAAA (2004). A total dose of 0.8 g/kg of 95% alcohol was administered to participants across a 2-h period. This dose was reduced by 8% for females to equate breath alcohol across gender (Hindmarch et al., 1991). The 0.8 g/kg was divided into four 8-oz cups with tonic water added to reach a total volume of 32 oz. Beverages were administered every 30 min to the participants. Consumption of the beverages was paced so that the participants had 10 min to consume the drink followed by a 20-min absorption period before consumption of the next drink (total time: 2 h). Breath alcohol concentrations (BrAC) were recorded every 20 min following the 2-h alcohol consumption period and at the completion of study participation, all participants were transported from the laboratory by a designated driver.
Measures
Impulsivity measurements
The BIS-11 (Patton et al., 1995) was administered to assess trait impulsivity. The BIS-11 has been used extensively to measure impulsive tendencies in both healthy and clinical populations. The BIS-11 is a 30-item self-report questionnaire assessing general impulsiveness. Items can be answered with one of four responses: 1, rarely/never; 2, occasionally; 3, often; and 4, almost always/always. A total score was obtained for responses on the BIS-11 30 items.
The GoStop Impulsivity paradigm (Dougherty et al., 2009) is a computer administered task that quantifies response inhibition as a behavioral measure of state impulsivity. Five-digit numbers in black are rapidly presented against a white computer screen for 500 ms, followed by a 1500-ms interstimulus interval. Participants are instructed to respond by pressing the left mouse button every time the number on the screen is identical to the previously presented number (go trials). Along with go trials, stop trials are also presented. A stop trial occurs when the five-digit number on the screen changes in color from black to red following a specified time delay (stop delay). The stop delays are 50, 150, 250 and 350 ms. A stop delay of 50 ms indicates that the five-digit number will appear in black for 50 ms, and then change to red for the remainder of the 500-ms presentation. Participants were instructed to withhold responding to any number that turns red while appearing on the screen (stop trials). The primary dependent variable was the 150-ms GoStop ratio. The 150-ms GoStop ratio was the number of incorrect responses to the 150-ms stop trials divided by correct responses to go trials. Greater impulsive behavior (defined as response inhibition failures) on this task is indicated by greater GoStop ratios. The 150-ms ratio has been shown to provide the best discrimination between high- and low-impulsive individuals (Marsh et al., 2002) and has shown sensitivity to the impairing effects of alcohol (Dougherty et al., 2008).
Subjective measurement of stimulation/sedation
To assess self-reported stimulation/sedation following alcohol administration, the BAES was used. The BAES is a visual analog scale composed of 14 descriptors of subjective feelings. Half of the descriptors are related to stimulant-like effects (talkative, up, elated, stimulated, vigorous, excited and energized), and the other seven are related to sedative-like effects (heavy head, sedated, slow thoughts, down, inactive, sluggish and difficulty concentrating). Each descriptor is rated by placing a mark along a 100-mm line anchored at the ends with the statements ‘not at all’ or ‘extremely’. Total stimulation and sedation scores were calculated by summing together all respective descriptor ratings. Additionally, to determine within individuals the relative amount of self-reported stimulation and sedation, a stimulation/sedation ratio was calculated.
Subjective measurement of mood
To assess self-reported mood states following alcohol administration, the POMS was used. The POMS is a 65-item questionnaire in which participants rate a series of mood states on a five-point Likert scale that ranges from ‘Not at all’ to ‘Extremely’. Six mood factors are identified including tension/anxiety, depression/dejection, anger/hostility, vigor, fatigue and confusion/bewilderment. A higher-order factor of arousal [(anxiety + vigor)–(fatigue + confusion); de Wit et al., 1999)] was determined from the POMS and used in the current study.
Data analyses
A forward stepwise regression was conducted with BAES stimulated/sedated ratio as the dependent variable. Ratios at each of the four delay intervals (50, 150, 250 and 350 ms) were independent variables. The goal of this analysis was to test whether the 150-ms interval ratio was the best predictor of BAES stimulated/sedated ratio. A series of regression analyses were then performed to (i) examine the relationship between impulsivity (150-ms interval ratio) and subjective intoxication effects (BAES stimulation and sedation) and (ii) examine the relationship between impulsivity (150-ms interval ratio) and subjective intoxication mood effects (POMS arousal). Preliminary analyses (independent samples t-test) were conducted to determine whether there were significant sex differences between ratings of BAES stimulation and sedation and POMS arousal following alcohol consumption. Initial analyses indicated that there were not significant sex differences in these measures and data were collapsed across sex for all subsequent analyses. Data analyses were conducted using the SigmaPlot version 11 software program (Systat Software Inc., San Jose, CA, USA). Statistical significance was defined as P < 0.05.
RESULTS
Group demographics
A total of 50 participants (34 males and 16 females) were included in this study. The mean age (± SD) of the sample was 26 ± 6 years of age and the mean IQ (± SD) was 112 ± 12. This population consumed on average (± SD) 13 ± 9 alcoholic drinks per week and had a mean (± SD) AUDIT score of 8 ± 3. The mean total BIS score (± SD) was 58 ± 9 for this group.
Breath alcohol concentrations
The drink administration procedure used in the study produced a peak breath alcohol of 0.08% (SD = 0.02%), which was achieved ∼20 min following completion of the alcohol administration procedure. This breath alcohol value is consistent with the NIAAA definition of a binge (2004), specifically 4–5 drinks in 2 h to produce a breath alcohol of 0.08%.
Impulsivity and subjective effects of alcohol
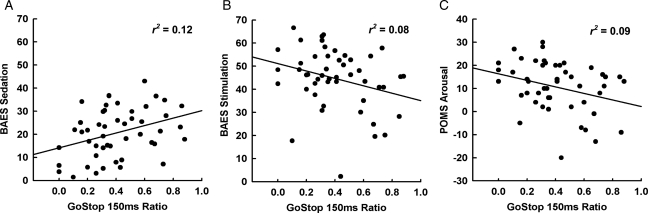
Multiple R was statistically significant for BAES stimulated/sedated ratio (F1,48 = 9.12, P = 0.004, R2= 0.16). As predicted, the 150-ms ratio was the only ratio of the four that contributed significantly to the BAES stimulation/sedation ratio (β = −0.40). In subsequent linear regression analyses, the 150 ms ratio was positively associated with BAES sedation (r2=0.12, b = 16.1, t(49) = 2.59, P = 0.13) and negatively associated with BAES stimulation (r2=0.08, b = −16.0, t(49) = −2.05, P = 0.046) and POMS arousal (r2=0.09, b = −14.2, t(49) = −2.19, P = 0.033) scores (Fig. 2).
Fig. 2.
Significant linear regressions between performance on the response-inhibition task and (A) self-reported stimulation as measured by the BAES; (B) self-reported sedation as measured by the BAES; and (C) self-reported arousal as measured by the POMS following alcohol consumption. Statistical significance was defined as P < 0.05.
Secondary analysis
As part of a secondary analysis exploring the relationship between a measure of trait impulsivity and subjective response to alcohol consumption, linear regressions were conducted between BIS-11 total score and BAES sedation and stimulation along with POMS arousal. There was not a significant relationship between the BIS total score and any of the subjective intoxication measures. Additionally, as there was a range of alcohol consumption within our sample (range: 2–39 drinks/week), we examined whether the level of alcohol consumption influenced our results. Linear regressions were conducted between drinks per week with subjective intoxication effects of alcohol (stimulation, sedation and arousal). Drinks per week was not a significant predictor of subjective response to alcohol in this population.
DISCUSSION
The data collected in this study, contrary to our hypothesis, suggested a negative relationship between impulsivity and stimulation following alcohol, such that individuals who performed more impulsively on a response-inhibition task reported the least stimulation following alcohol consumption. Additionally, there was a negative relationship between impulsive behavior and arousal following alcohol consumption, with individuals who performed more impulsively also reporting less arousal following alcohol consumption. These data suggest that individuals with lower levels of state impulsivity actually endorse more reinforcing effects (i.e. arousal and stimulation) following alcohol consumption compared with higher impulsive individuals, contrary to what was hypothesized.
Previous research has suggested that aggressive impulsive individuals are under aroused at rest, but—in response to photic stimulation—show increased arousal (as measured by evoked electrical potentials) compared with non-aggressive controls (Houston and Stanford, 2001). Although our results fail to show greater arousal in impulsive individuals following alcohol administration, methodological differences between studies (objective physiological measure versus subjective measures) and the possible interaction between impulsivity and aggression in the previous study may have led to divergent findings. The results of this study do suggest that individuals with high levels of impulsive behavior may be less sensitive to the stimulating effects of alcohol, and need more alcohol to experience the same level of stimulation as their less impulsive counterparts. To our knowledge, there has not been a study that has directly tested and confirmed this hypothesis in human populations. However, animal studies have suggested that more impulsive animals are less sensitive to motor stimulation following alcohol administration (Mitchell et al., 2006). Additionally, similar results have been found in human studies, although with amphetamine rather than alcohol. In these studies, individuals with higher lapses of attention (a proposed dimension of impulsivity; de Wit, 2009) reported less liking and desire for more of the drug following amphetamine administration (McCloskey et al., 2010). Those who perform more impulsively on a response-inhibition task following acute alcohol administration consumed more alcohol in an ad lib consumption procedure (Weafer and Fillmore, 2008), perhaps to experience the positive stimulating effects of alcohol. A positive correlation exists between impulsivity and consumption of alcohol (Grau, 1999). Impulsive individuals may need to consume more alcohol per drinking occasion to achieve the stimulating effects of alcohol. Future studies are needed that assess the relationship of impulsivity and subjective response to alcohol using a full dose range of alcohol.
The time points at which the subjective effects of stimulation and sedation following alcohol consumption were measured differed between this study and previous studies. Previous studies measured stimulation and sedation at multiple time points within the range of 10–60 (Thomas et al., 2003), 15–165 (King et al., 2002) and 30–180 min (Holdstock et al., 2000) following the first sip of alcohol. This study measured stimulation and sedation (BAES) at only one time point, which was 50 min following completion of alcohol consumption (Fig. 1; corresponding to 170 min following first sip of alcohol). At this time point, the mean breath alcohol (± SEM) for the group was ∼0.08% ± 0.01. A differentiator model suggests that individuals who are at risk for alcohol problems experience the greatest stimulation during the ascending limb of the breath alcohol curve and fewer sedative-like effects during the descending limb (Newlin and Thomson, 1990), suggesting that the subjective effects of stimulation and sedation can vary according to the time points on the breath alcohol curve in which these measurements are captured. Although stimulation and sedation were measured following the peak of the breath alcohol curve, measures of mood (POMS) were collected closer to the peak of the breath alcohol curve (20 min following alcohol administration; Fig. 1) and revealed a negative association with impulsivity and arousal. Perhaps, if subjective ratings of stimulation and sedation following alcohol consumption were collected at additional time points along the breath alcohol curve, a different directional relationship between impulsivity and subjective effects of alcohol would have emerged.
It is of interest that state impulsivity, but not trait impulsivity, predicted sensitivity to the stimulating effects of alcohol. The differentiation between the predictive values of trait versus state impulsivity in this context may be due to either less self-reporting of impulsive behaviors or the selection of non-problematic alcohol drinkers. Perhaps higher levels of trait impulsivity would have been found if the sample included individuals with a more extensive drinking history or levels of alcohol use disorders. Previous studies have shown that high levels of trait impulsivity are found in those populations who suffer from alcohol dependence (Bjork et al., 2004; Mitchell et al., 2005). Future studies to determine the role of trait impulsivity and sensitivity to the subjective effects of alcohol need to be conducted, perhaps in populations who suffer from alcohol-use disorders, to understand the interactions between these risk factors further.
Previous studies that have found increased sensitivity to the stimulating effects of alcohol have observed this phenomenon in heavy drinkers, alcoholics or individuals with a family history of alcohol problems (Holdstock et al., 2000; King et al., 2002; Schuckit, 1994; Thomas et al., 2003). Two studies in which heavy drinkers reported greater stimulation following alcohol consumption had individuals who averaged roughly 12–24 drinks per week and engaged in approximately 45 binge drinking episodes across a 6-month period (Holdstock et al., 2000; King et al., 2002). Participants in this study drank on average 12 drinks per week and engaged in approximately four binge drinking episodes across a 3-month time span. As part of inclusion criteria for this study, an AUDIT cutoff of 12 was imposed. This cutoff was chosen as it has previously been shown as adequate for excluding individuals who may be dependent upon alcohol (Conigrave et al., 1995). By employing this AUDIT cutoff, we may have excluded individuals with the highest risk for developing alcohol dependence. If individuals at higher risk for alcohol dependence were included in the sample, the hypothesized increased sensitivity to the stimulating effects of alcohol in higher impulsive individuals may have been observed. It should be noted though that we did not find any significant relationships between level of alcohol consumption (drinks per week) and subjective responses to alcohol (sedation, stimulation and arousal) in this sample.
Another important consideration when comparing the results from this study to the aforementioned studies is the pacing of alcohol administration. The present study administered a 0.8 g/kg dose of alcohol in a binge-like fashion, in which participants consumed four alcoholic drinks over a period of 2 h, which corresponded to BrAC of 0.08%. The aforementioned studies administered doses of alcohol that ranged from 0.4 to 0.8 g/kg consumed within 10–25 min (Holdstock et al., 2000; King et al., 2002; Thomas et al., 2003). While administering alcohol in a binge-like manner (according to the NIAAA definition of a binge drinking episode) resulted in breath alcohol curves similar to bolus administration of alcohol, consuming alcohol over a longer time period (2 h versus 10–25 min) may affect the subjective effects of alcohol. There is evidence to suggest that the pacing of drink administration influences the subjective effects of alcohol, such that stimulant effects increase with speed of consumption (Morean and Corbin, 2010). The BAES measurements were recorded when BrAC were between 0.07 and 0.08%, though the slower absorption and slower onset of the pharmacological effects may have led to differences in reports of stimulation and sedation compared with studies that employed a bolus dosing procedure. The differences in subjective effects when alcohol is administered in a bolus dose versus multiple doses over a longer time period has not been well characterized and represents a question that warrants additional research.
In summary, results from this study suggest that with respect to impulsivity, a significant positive relationship with self-reported sedation and a significant negative relationship with self-reported stimulation and arousal following alcohol consumption exists. These findings may suggest that individuals with high levels of impulsive behavior are less sensitive to the stimulation effects of the dose of alcohol (0.8 g/kg) administered in this study. While a decreased sensitivity to the stimulating effects of alcohol may lead impulsive individuals to consume higher doses of alcohol to achieve the desired effect, the data from this study cannot fully support that assumption. Future studies should be aimed at assessing higher doses of alcohol and subjective stimulation at multiple time points along the breath alcohol curve in impulsive individuals. Also, future studies should examine whether the relationship between impulsivity and subjective response to alcohol differs in those individuals with a family history of alcohol problems. As it stands, the results from this study suggest that these impulsivity-based differences in the subjective effects of alcohol may reflect differing motivations for alcohol use. Understanding these motivations may provide insight on how to reduce risky drinking in impulsive populations.
FUNDING
This work was supported by a grant from National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (AA017056). Authors E.E.S. and K.A.B.-S. were supported by an institutional training grant from National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (AA07565).
Conflict of interest statement: none declared.
REFERENCES
- Babor TF, Higgins-Biddle JC, Saunders JB., et al. The Alcohol Use Disorders Identification Test (AUDIT) Manual. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ., et al. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–50. doi: 10.1016/j.alcohol.2004.06.012. doi:10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Brook JS, Whiteman M, Cohen P., et al. Longitudinally predicting late adolescent and young adult drug use: childhood and adolescent precursors. J Am Acad Child Adolesc Psychiatry. 1995;34:1230–8. doi: 10.1097/00004583-199509000-00022. doi:10.1097/00004583-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol use disorder identification test. Addiction. 1995;90:1349–56. doi: 10.1046/j.1360-0443.1995.901013496.x. doi:10.1111/j.1360-0443.1995.tb03552.x. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. doi:10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Svenson J, York A. Non-specific effect of naltrexone on ethanol consumption in social drinkers. Psychopharmacology (Berl) 1999;146:33–41. doi: 10.1007/s002130051085. doi:10.1007/s002130051085. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES., et al. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–20. doi: 10.1016/j.drugalcdep.2008.02.002. doi:10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM., et al. Dinstinctions in behavioral impulsivity: implications for substance abuse research. Addict Disord Their Treat. 2009;8:61–73. doi: 10.1097/ADT.0b013e318172e488. doi:10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine M. Anticipated biphasic effects of alcohol vary with risk for alcoholism: a preliminary report. Alcohol Clin Exp Res. 1994;18:711–4. doi: 10.1111/j.1530-0277.1994.tb00935.x. doi:10.1111/j.1530-0277.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits? Alcohol Clin Exp Res. 2003;27:44–50. doi: 10.1097/01.ALC.0000047300.46347.CE. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. doi:10.1037/1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M., et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-patient Edition. New York, New York: Biometrics Research Department; New York State Psychiatric Institute; 2002. [Google Scholar]
- Grau EOG. Personality traits and alcohol consumption in a sample of non-alcoholic women. Pers Individ Dif. 1999;27:1057–66. doi:10.1016/S0191-8869(99)00047-1. [Google Scholar]
- Hindmarch I, Kerr JS, Sherwood N. The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol Alcohol. 1991;26:71–9. [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–94. doi:10.1111/j.1530-0277.2000.tb02057.x. [PubMed] [Google Scholar]
- Houston RJ, Stanford MS. Mid-latency evoked potentials in self-reported impulsive aggression. Int J Psychophysiol. 2001;40:1–15. doi: 10.1016/s0167-8760(00)00120-3. doi:10.1016/S0167-8760(00)00120-3. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005;69:353–73. doi: 10.1016/j.biopsycho.2004.08.004. doi:10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A., et al. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. doi:10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H., et al. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–35. [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA., et al. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207:163–72. doi: 10.1007/s00213-009-1645-x. doi:10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M., et al. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology (Berl) 2005;182:452–9. doi: 10.1007/s00213-005-0116-2. doi:10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Marsh DM, Dougherty DM, Mathias CW., et al. Comparison of women with high and low trait impulsivity using response-disinhibition and reward choice. Pers Individ Dif. 2002;33:1291–310. doi:10.1016/S0191-8869(02)00014-4. [Google Scholar]
- Martin CS, Earleywine M, Musty RE., et al. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. doi:10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCloskey M, Palmer AA, de Wit H. Are attention lapses related to d-amphetamine liking? Psychopharmacology (Berl) 2010;208:201–9. doi: 10.1007/s00213-009-1719-9. doi:10.1007/s00213-009-1719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Dropplemen LF. Profile of Mood States Manual. San Diego: Educational and Teaching Services; 1971. [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M., et al. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N., et al. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–37. doi: 10.1111/j.1530-0277.2006.00047.x. doi:10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 34:385–95. doi: 10.1111/j.1530-0277.2009.01103.x. doi:10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L., et al. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26:1299–306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–5. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. doi:10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- NIAAA. 2004. In Newsletter Vol. Winter, p. 3.
- Noel X, Bechara A, Dan B., et al. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–86. doi: 10.1037/0894-4105.21.6.778. doi:10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38:151–6. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. doi:10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C., et al. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcohol Clin Exp Res. 1996;20:1542–52. doi: 10.1111/j.1530-0277.1996.tb01697.x. doi:10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Poikolainen K. Risk factors for alcohol dependence: a case–control study. Alcohol Alcohol. 2000;35:190–6. doi: 10.1093/alcalc/35.2.190. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Blekher T., et al. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1320–30. [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the experiential discounting task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. doi:10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH., et al. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–43. doi: 10.1901/jeab.1999.71-121. doi:10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Grunsell L. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcohol Clin Exp Res. 2008;32:1096–104. doi: 10.1111/j.1530-0277.2008.00672.x. doi:10.1111/j.1530-0277.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Simons JS, Carey KB, Gaher RM. Lability and impulsivity synergistically increase risk for alcohol-related problems. Am J Drug Alcohol Abuse. 2004;30:685–94. doi: 10.1081/ada-200032338. doi:10.1081/ADA-200032338. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Voronin K., et al. Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol. 2003;65:330–5. doi: 10.15288/jsa.2004.65.330. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC., et al. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91:213–9. doi: 10.1016/j.drugalcdep.2007.05.025. doi:10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. doi:10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lozano O, Moya M., et al. Psychometric properties of a Spanish version of the UPPS-P impulsive behavior scale: reliability, validity and association with trait and cognitive impulsivity. J Pers Assess. 2010;92:70–7. doi: 10.1080/00223890903382369. doi:10.1080/00223890903382369. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology (Berl) 2008;201:315–24. doi: 10.1007/s00213-008-1284-7. doi:10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–7. doi: 10.1037/1064-1297.11.3.210. doi:10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]