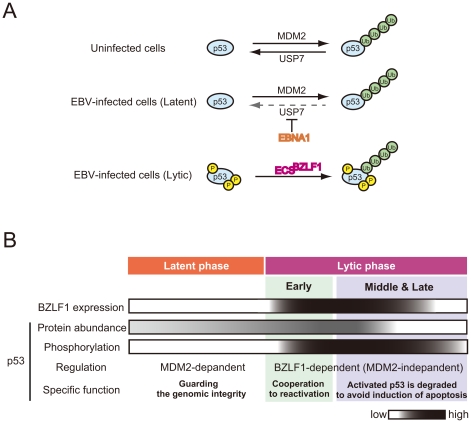
Figure 1. Stage-specific regulation of p53 during EBV infection.
(A) The ubiquitination of p53 is regulated by both MDM2 E3 ligase and USP7 deubiquitinase in uninfected cells. During EBV latent infection, EBV latent EBNA1 protein inhibits USP7 and thereby drives the ubiquitination of p53. Phosphorylated p53 is ubiquitinated by BZLF1 protein-associated E3 ligase independently of MDM2 during lytic infection. (B) During the latent phase of EBV infection, p53 is quantitatively regulated by MDM2 ubiquitin ligase via the ubiquitin-proteasome pathway [36], serving as a guardian of genome stability. Expression of BZLF1 protein induces virus-productive (lytic) replication through the ordered cascades of viral gene expression, and concomitantly host DNA damage responses [9], leading to p53 phosphorylation and release of p53 from the MDM2-dependent regulation [36]. In the early stages of lytic infection, the inactive (hypophosphorylated) form of p53 cooperates with viral factors including BZLF1 protein to stimulate virus replication [26], [27]. In the middle and late stages of infection, active (hyperphosphorylated) p53 is ubiquitinated by BZLF1 protein–associated ECS ubiquitin ligase complexes and is degraded in a proteasome-dependent manner to inhibit apoptosis [37].