Abstract
There are well-recognized sex differences in many pituitary endocrine axes, usually thought to be generated by gonadal steroid imprinting of the neuroendocrine hypothalamus. However, the recognition that growth hormone (GH) cells are arranged in functionally organized networks raises the possibility that the responses of the network are different in males and females. We studied this by directly monitoring the calcium responses to an identical GH-releasing hormone (GHRH) stimulus in populations of individual GH cells in slices taken from male and female murine GH-eGFP pituitary glands. We found that the GH cell network responses are sexually dimorphic, with a higher proportion of responding cells in males than in females, correlated with greater GH release from male slices. Repetitive waves of calcium spiking activity were triggered by GHRH in some males, but were never observed in females. This was not due to a permanent difference in the network architecture between male and female mice; rather, the sex difference in the proportions of GH cells responding to GHRH were switched by postpubertal gonadectomy and reversed with hormone replacements, suggesting that the network responses are dynamically regulated in adulthood by gonadal steroids. Thus, the pituitary gland contributes to the sexually dimorphic patterns of GH secretion that play an important role in differences in growth and metabolism between the sexes.
Keywords: sex hormones, body growth, calcium signaling, systems biology
In most species, males and females display a marked phenotypic divergence in body size, with increased growth rate and body mass being a predominantly masculine trait. Furthermore, in all species examined to date, the growth hormone (GH) axis demonstrates sex-specific differences in hormone contents, secretory outputs, and secretory patterns (1) and their effects on gene expression (2–4). The secretion of GH is controlled by hypothalamic GH-releasing hormone (GHRH) and somatostatin, and there is good evidence for sex-specific imprinting on hypothalamic hypophysiotropic neurons exerted by gonadal steroid exposure early in life (5), with ongoing effects during puberty (6). This has led to the conclusion that the sexually dimorphic control of GH patterns reflects sex differences in GHRH and somatostatin inputs to the pituitary gland. Acute changes in gonadal steroid environment drastically alter the patterns of GH pulsatility in adulthood (7, 8); however, although they receive sexually dimorphic inputs (9, 10), GHRH neurons do not display sex-specific electrical characteristics (9, 11). We have previously shown that GH cells in the male mouse pituitary gland form an extensive homotypic cell network with an architecture that exhibits marked plasticity during sexual maturation and that can be altered by gonadectomy (12). Thus, it was important to determine whether male and female pituitary glands would show different responses to the same stimulus in the absence of any hypothalamic influence. To explore this, we assessed the functional activity of multiple GH cells within the network by monitoring calcium spikes (13, 14) in identified populations of individual GH cells in pituitary slices taken from male and female GH-eGFP transgenic mice (15), intact or following gonadectomy, with or without acute gonadal steroid replacement. Our results show that the same GHRH challenge elicited strikingly different patterns of activation of GH cells from male and female glands. This effect was not due to a permanent alteration in GH cell network architecture, and it was not observed when GHRH responses were monitored in populations of isolated male or female GH cells studied in vitro or in vivo. However, the sexually dimorphic responses could be switched back and forth by gonadectomy and steroid hormone replacement, respectively. Thus, the functional organization of the pituitary makes a significant contribution to the sexually dimorphic responses in the GH axis.
Results
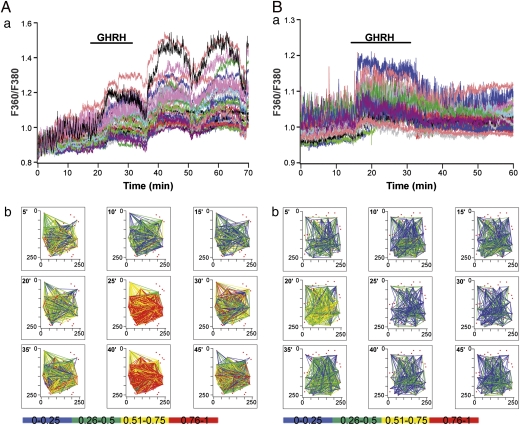
Monitoring of the functional activity of GH cells in slices loaded with calcium indicator taken from male and female GH-eGFP mice revealed no differences in the proportion of cells showing spontaneous activity (59.9% of active cells in males and 60% in females; n = 815 and 426 cells, respectively). In contrast, striking differences were seen in their responses to a GHRH stimulus (Fig. 1). In males, ∼60% of GH cells responded to GHRH with coordinated increases in cell activity (n = 16 pituitary slice fields). In some slices, a single GHRH exposure generated repetitive waves of calcium spikes that persisted for long periods even after cessation of secretagogue application (e.g., Fig. 1Aa; ref. 12). Interestingly, a relationship was noted between the incidence of calcium waves and the location of GH cells recorded. No GHRH-induced repetitive trains of calcium spikes were observed in medial regions of coronal pituitary slices, as we reported previously (12); however, GHRH triggered a cyclical increase in spike firing in the lateral wings of the pituitary, with increased prevalence in regions closer to the medial strip (six of nine recorded fields; n = 9 animals; Fig. 1Aa; ) versus those at the wing extremity (two of seven recorded fields; n = 5 animals). Fig. S1 shows a representative example of a noncycling calcium response observed in a male animal.
Fig. 1.
GHRH triggers differential calcium responses in male and female pituitary slices. (Aa and Ba) Monitoring of calcium responses to GHRH (10 nM) in GH-GFP cells in male (A) and female (B) pituitary slices loaded with the fluorescent calcium dye fura-2. (Ab and Bb) Correlation maps, calculated at 5-min intervals, identifying significantly correlated GHRH-responsive GH-GFP cell pairs (Pearson R). Red dots show the location of all GHRH-responsive GH cells. Significantly correlated cell pairs are connected with a straight line, the color of which represents the correlation strength; the bottom bar provides the color code. Recorded fields were located in the lateral regions of the pituitary gland close to the median strip.
In females, only ∼35% of GH cells responded to GHRH (P < 0.05 compared with males). Unlike in males, the nature of these responses was similar irrespective of the location of recorded GH cells, with rapid diminution of calcium spiking activity following withdrawal of stimulus. No waves of calcium spikes were observed in slices taken from females (seven recorded fields; n = 7 animals) (Fig. 1Ba).
The GH network activity in males and females is depicted in Fig. 1 Ab and Bb as the likelihood of positive correlation in activity between identified pairs of GH cells, with “heat maps” indicating the strength of correlation between pairs. Movie stacks showing these heat maps at a larger scale, provided in Movies S1 and S2, clearly illustrate the repetitive waves of coordinated activity observed in some male slices and demonstrate that the same pairs of cells tended to be cyclically responsive to a single activation of GHRH. Although females did not display this cyclic response, providing repeated GHRH stimuli to female glands caused the majority of GH cells that responded to the first GHRH stimulus to respond to the second stimulus as well (72% ± 3%; n = 4) (Fig. S2). Interestingly, although a similar total number of cells responded to the second stimulus, some previously responding cells did not respond a second time, whereas others responded only to the second stimulus. We also found that GHRH receptor expression in GH-eGFP cells, as measured by quantitative PCR (qPCR), did not differ between the sexes (relative expression, 2.95 ± 0.86 for males and 2.16 ± 0.47 for females; n = 3 for each condition; P > 0.05).
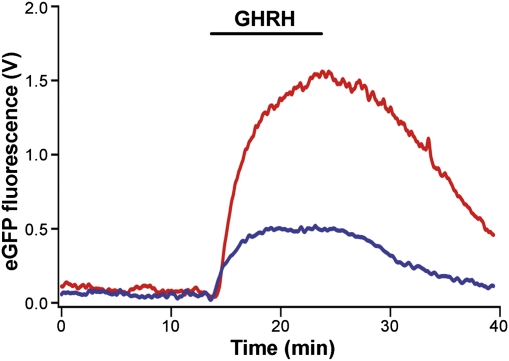
To test for sex differences in secretory output of GH from pituitary slices, we took advantage of the fact that in GH-eGFP mice, the eGFP fusion product is targeted to GH secretory vesicles and is cosecreted with endogenous GH in a calcium-dependent manner (15). As measured using an online fluorescence detector coupled to a slice perfusion chamber containing a GH-eGFP pituitary slice, the eGFP fluorescence secretory responses to GHRH were significantly larger (peak values, P < 0.05) and their time-to-peak more prolonged in males than in females (10.9 ± 0.7 min vs. 7.3 ± 1.0 min; P < 0.05; n = 6 slices for each condition) (Fig. 2).
Fig. 2.
GHRH triggers differential secretory responses in male and female GH-eGFP pituitary slices. Online fluorescence monitoring of eGFP release from pituitary slices from male (red trace) and female (blue trace) transgenic mice expressing eGFP specifically targeted to the GH secretory vesicles. GHRH was introduced into the perfusate at 10 nM, as indicated by the horizontal bar. Data shown are the average from six slices for each sex.
We tested the possibility that these sex differences in response might be explained by a morphological difference in the GH network architecture between adult males and adult females. Both female and male mice (Fig. S3, Upper) exhibited GH cell clusters interlinked with cell strands. As reported previously (12, 16), GH cell clustering, as reflected in the volume/surface ratio of the GH cell network, increased transiently at puberty in males. We were not able to show a similar increase in females during sexual maturation (Fig. S3, Lower). This indicates a difference at puberty between males and females, but no persistent structural differences in the GH cell network architecture in adult males and adult females.
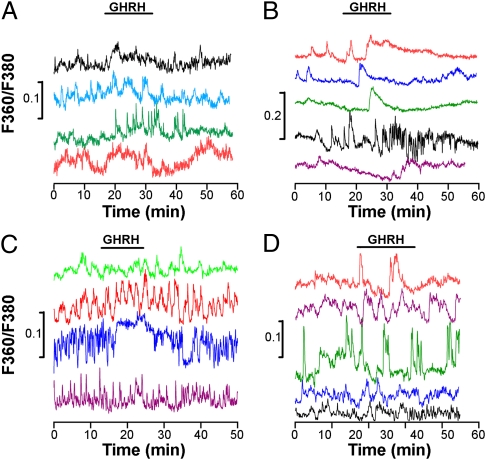
To test whether sex differences are intrinsic to individual GH cells, we examined calcium activity and responses to GHRH in identified GH cells isolated from male or female pituitary glands. We observed asynchronous, variable GHRH calcium response profiles in male and female GH cells, with no sex difference in the proportion of responsive isolated cells (16% of GHRH-responsive cells in males and 13% in females; n = 217 and 272 recorded cells, respectively; P > 0.05) (Fig. 3 A and B). To test whether this also holds true for cells isolated in situ, we used a double-transgenic model that maintains small numbers of isolated, GHRH-responsive GH-eGFP cells in the pituitary gland (17) in a GHRH-deficient background (GHRH-M2) (18). Fig. 3 C and D shows calcium responses to GHRH in single GH cells in slices from these male and female GHRH-M2 × GH-eGFP mice. Although the GH cells displayed spontaneous calcium spikes and prolonged increases in spike frequency in response to GHRH, there was no evidence of coordinated cell–cell activity and no evident sex differences (22% of GHRH-responsive cells in males and 30% in females; n = 89 and 63 recorded cells, respectively; P > 0.05). Taken together, the results from these two experimental approaches suggest that the sex differences observed in male and female pituitary GH responses to GHRH in situ require an intact GH cell network.
Fig. 3.
GH cell network disruption precludes the sexually dimorphic trait of GHRH calcium responses in GH-eGFP cells. (A and B) Asynchronous calcium responses to GHRH (10 nM) in GH-eGFP cells enzymatically isolated from male (A) and female (B) pituitary glands and dispersed onto coverslips. (C and D) Asynchronous calcium responses to GHRH (10 nM) in single GH-eGFP cells in situ in male (C) and female (D) GH-GFP x GHRH-M2 mice. In both cases, the correlation (R) value after application of GHRH was no different from that observed under control conditions.
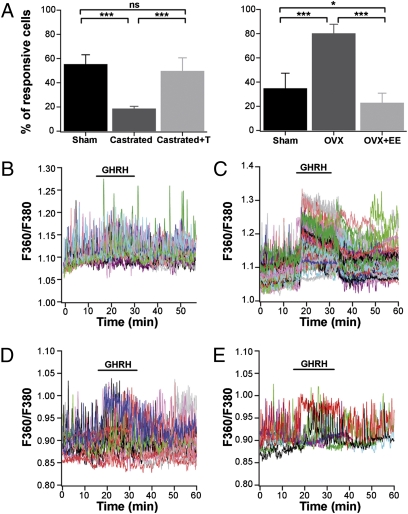
What might cause these sex differences at the pituitary level? One obvious factor is the differing sex steroid environments in adult males and adult females, given the known capacity of gonadal steroids to alter GH patterns in adult animals (19). To investigate whether sex steroids could regulate these sexually dimorphic responses, we performed experiments on slices taken from male and female gonadectomized mice, with or without sex hormone replacement. Slices from 60-d-old males (castrated 15 d earlier) exhibited fewer (∼20%) GHRH-responsive cells compared with control sham-operated males; testosterone supplementation in castrated males restored the proportion of GHRH-responsive cells back to that seen in normal males (Fig. 4A, Left). In gonadectomized males, the initial responses to GHRH still showed cell–cell coordination at the onset of GHRH application (Fig. 4B). The waves of activity detected in intact males following GHRH exposure (Fig. 1Aa) were lost after gonadectomy (Fig. 4B), and were not restored in animals given replacement therapy (Fig. 4D).
Fig. 4.
GHRH calcium responses in the GH cell network are amenable to sexual hormone manipulation in adulthood. (A) Proportion of GHRH-responsive cells in sham-operated and gonadectomized male (Left) and female (Right) mice, treated with and without hormone replacement. Data are mean ± SD. ***P < 0.001; *P < 0.05; ns, nonsignificant. (B and C) Representative calcium traces of GH-GFP cell responses to GHRH in pituitary slices obtained from gonadectomized male (B) and female (C) mice. (D and E) Representative calcium traces of GH-GFP cell responses to GHRH (10 nM) in pituitary slices obtained from a castrated male supplemented with testosterone (D) and an ovariectomized female supplemented with ethinyl estradiol (E).
Conversely, slices from 60-d-old females ovariectomized 15 d earlier demonstrated a significant increase in the proportion of GH cells responding to GHRH (∼80%), and estrogen replacement reversed this effect (Fig. 4A, Right). In ovariectomized females, GH cells showed coordinated activity in response to GHRH, but no repetitive calcium waves were observed with or without estrogen replacement (Fig. 4 C and E).
In gonadectomized males with testosterone supplementation, the initial responses to GHRH still showed cell–cell coordination at the onset of GHRH application, but the prolonged waves of calcium spiking activity observed post-GHRH application in some intact males could not be reproduced by this acute hormone replacement protocol, even when cytosolic calcium fluctuations were monitored in lateral regions of the pituitary gland (Fig. 4D). It has been shown that androgen replacement in gonadectomized males can fail to restore some characteristic features of male GH pulses, such as prolonged duration (20, 21). Thus, we decided to test whether a male-like response pattern could be obtained by masculinizing ovariectomized females using acute testosterone supplementation. Indeed, this has been demonstrated to be highly effective in promoting male-like GH pulse patterns in female rodents (8). Under these conditions, ∼60% of GH cells responded to GHRH, similar to the findings in intact males (n = 8 slices). Even more striking, cells located in the lateral regions close to the medial strip were able to generate bouts of coordinated calcium spiking activity, albeit with shorter durations than these observed in intact males (5/8 recorded fields) (Fig. S4).
Discussion
Our findings demonstrate that the pituitary GH network responses to GHRH are sexually dimorphic in adulthood. When challenged by the same GHRH input, responsive GH cells in males and females differ substantially in the extent of coordinated firing and GH output (as inferred from their GFP output). Because we did not see these differences when we tested GH cells in isolation, we conclude that they are a property of the network that GH cells form in the pituitary gland. The major sexual dimorphic feature is that the proportion of responsive GH cells differed significantly between the sexes. The resolution of our methods allowed us to identify the individual responding cells, and demonstrated that our results did not reflect a random population of cells or a strictly defined subset of responsive GH cells. Indeed, a consecutive GHRH challenge failed to elicit a second response in some cells, but did elicit a response in some initially nonresponsive cells. This is entirely consistent with earlier observations on variable responses in individual GH cells, and also with the finding of greater secretion in response to GHRH in individual male GH cells compared with female cells (22). The proportion of responsive cells was sensitive to the prevailing gonadal steroid environment; it could be switched by gonadectomy and reversed by acute hormone replacement. This suggests dynamic regulation of the GH cell network response by gonadal steroids in both males and females.
GH Cells in an Intact Network Show Sex-Specific Organized Patterns of Response to GHRH.
There is much evidence indicating that individual GH cells isolated from their tissue context display stochastic cell signals due to spontaneous electrical activity (23). In situ, the GH cell network largely retains the stochastic nature of spontaneous calcium signals under basal conditions. We have previously shown that only a few neighboring GH cells are tightly synchronized in the absence of a GHRH input (12, 24). Strikingly, following challenge with GHRH, GH cells responded in a tightly coordinated manner. The GH cell network structure was essential for this temporally correlated response, as evidenced by the stochastic behavior of dissociated GH cells even in the presence of GHRH. This was not simply the result of harsh isolation conditions; GH cells in transgenic mice with a severely disrupted GH cell network (17, 18) also did not exhibit coordinated responses to exogenous GHRH. These results illustrate that the presence of a topologically arranged network is critical for the propagation of information among the GH cells. We suggest that the network serves to amplify the secretory response of the GH cell population by coordinating a coherent GH pulse in response to GHRH. When cells respond as individual isolated units, this potentially can activate pathways that compromise cell activity and survival (25).
What is the mechanistic link between the coordinated trains of calcium spikes induced by GHRH and GH secretion in vivo? GHRH-induced GH secretion depends on calcium entry (15, 26, 27), which in endocrine cells occurs during action potential firing (12, 23, 28). Trains of calcium spikes, as induced by GHRH in both male and female pituitary slices, are considered one of the most effective patterns of episodic calcium entry in triggering exocytosis in various neuroendocrine/endocrine tissues (29, 30), including pituitary cells recorded in situ (31). In our study, eGFP (GH) release in response to GHRH stimulus was greater in slices from male pituitary glands than in slices from female glands. Given the higher proportion of GH cells responsive to GHRH in males, simple summation of the ensuing calcium-dependent exocytotic responses would account for the observed differences in GH secretion (32–34). Finally, a remarkable feature of GH responses in males was that a single application of GHRH could trigger repetitive waves of calcium spiking activity that persisted well beyond the cessation of GHRH exposure. This effect, which was not seen in females, might contribute to the delayed time to peak of the secretory response detected in males. Rhythmic calcium waves may trigger other functions as well, such as gene expression (35), because cell calcium spiking activity influences a wide range of downstream processes depending on the amplitude of the calcium spikes, as well as their frequency and patterns of discharge (13, 14).
Further studies are needed to investigate how and why only a portion of the GH cell network, spatially restricted within the lateral pituitary wings, is able to generate repetitive trains of calcium spiking activity in response to GHRH input. One possible explanation is that an enhanced information flow exists close to the medial portion of the pituitary gland due to increased cell–cell connectivity/communication. It also should be noted that our results reflect only those GH cells responding to GHRH with calcium spikes. Most, if not all, GH cells express GHRH receptors (36), and we found that some initial nonresponders were responders to a second GHRH pulse. The cells that do not respond to GHRH with a calcium spike might activate other pathways, such as cAMP (37).
GH Cell Network Activity Is Regulated by Gonadal Steroid Hormones in Adulthood.
Despite the fact that adult males and adult females exhibited no permanent difference in the organization of GH cell network architecture, their GH cells generated markedly different activity in response to GHRH in situ. Furthermore, this activity could be manipulated by changing the gonadal steroid levels. These effects of gonadal steroids on GH network performance in adulthood are entirely consistent with the results of previous in vivo studies of GH pulsatility (19, 38). Sex-specific traits are controlled by both genetic (39) and endocrine (gonadal steroid) mechanisms (40, 41), and include sex-specific effects on transcription in the pituitary gland (42). It seems unlikely that this sexual dimorphism of GH cell network responsiveness is mediated by sex-determination genes independently of gonadal steroid environment (40, 43–45), given that the female GH cell network can be readily induced to display male-like proportions of responding cells, and vice versa, simply by manipulating the sex steroid environment. Moreover, a fine balance of sex hormones is likely required for the generation of repetitive waves of calcium spikes in response to a GHRH challenge, given that such recurrent waves were observed only in postpubertal ovariectomized females treated with testosterone, an experimental condition known to profoundly “masculinize” GH pulse rhythms in adult female rodents (8). Finally, we do not believe that these sex-specific responses reflect ultra-rapid effects of steroids, because slices from both males and females maintained their differential responses for several hours when isolated in media lacking steroids. Further investigation of how the sex hormone environment alters the likelihood of GHRH responsiveness or coordinated activity in vivo is needed.
In invertebrates, such as fruit flies, male courtship depends on the concerted activities of sexually dimorphic neuronal networks in both the mushroom body (46) and peripheral tissues (39, 47). In higher organisms, gonadal hormones are known to exert organizational effects on brain circuits (48). The sex determination of both behavioral and somatic features has been attributed to sexually dimorphic nervous system circuits, such as the vomeronasal system for male or female behaviors (40), the sexually dimorphic nucleus in the rodent hypothalamus for control of gamete release and body growth at sexual maturation (40, 49), and the spinal nucleus of the bulbocavernosus, which innervates the striated muscles mediating reflex erectile function (50). Classical studies in rats (20, 21, 49, 51) have shown that exposure to androgens and estrogens during a critical perinatal window permanently imprints a specific neuronal architecture that subsequently drives sex-specific behavioral and somatic traits. Along with these “organizational effects” are reversible changes induced by steroids in adulthood (“activational effects”) (52, 53). Gonadal steroid regulation of GH patterns has largely been attributed to effects on the hypothalamic pulse generator, although it is well recognized clinically that sex steroid “priming” in prepubertal children radically alters the pituitary responsiveness to GH stimulation tests (54). Although some of these activational effects may reflect direct effects on GH or GHRH gene expression, our data indicate that gonadal steroids also regulate the pattern and magnitude of the GH response in adulthood at the pituitary level.
In summary, mice express a sexually dimorphic GH cell pituitary network that contributes to the shaping of highly ordered sex-specific GH pulses generated in response to physiological demands. The hypothalamic somatostatin and GHRH systems generally considered responsible for generating sexually dimorphic GH secretory patterns signal to target GH cells, which respond in a sexually dimorphic manner during adulthood.
Materials and Methods
Animals.
Male and female 2- to 3-mo-old GH-eGFP mice were generated and used as described previously (15). Some animals were crossed with GHRH-M2 mice (18), and their double-transgenic progeny were used. For gonadectomy experiments, 45-d-old mice were anesthetized by an i.p. injection of a ketamine (1%)/xylazine (0.1%) mixture. Three groups of animals were studied: sham-operated, gonadectomized, and gonactomized with hormone replacement (testosterone propionate, 1.2-mg pellet, or ethynylestradiol, 0.03-mg pellet; Innovative Research of America) (55, 56). Experiments were carried out 15 d after surgery. All animal studies were conducted in compliance with the animal welfare guidelines of the European Community and/or the United Kingdom Home Office, as appropriate.
Multiphoton Imaging, eGFP Release Assays, Calcium Recording, and Real-Time qPCR.
In paraformaldehyde-fixed pituitaries, two-photon excitation microscopy was used to image the location of GH-eGFP cells in three dimensions, as described previously (12). Volume/surface ratios of 3D GH cell network structures were quantified using the Imaris surface rendering tool (Bitplane). GH release from perfused pituitary slices was monitored by continuous real-time monitoring of the secretion of vesicle-targeted eGFP (15). Using an adapted Zeiss upright microscope equipped with a photomultiplier tube, fluorescence outflow was measured directly as it passed through a reflective perfusate chamber. For multicellular cytosolic calcium measurements, GH-eGFP cells loaded with the fluorescent calcium dye fura-2 AM (Molecular Probes) were identified as described previously (15). Usually one, and rarely two, planar slices per pituitary gland were used, and calcium measurements were made no more than 2–8 h postmortem. Ratiometric cytosolic calcium fluctuations were monitored using a fluorescent stereomicroscope (StereoDiscovery; Zeiss) equipped with a water-immersion Zeiss objective (W Plan-Apochromat, 20×, 1.0 NA). Fluorescence excitation was alternately delivered at 360 nm and 380 nm by a Lambda LS 300-W xenon arc lamp (Sutter Instrument) fitted with a fast-rotating filter wheel (27-ms lag) and linked to the stereomicroscope with an optical fiber. Fluorescence emissions were captured (360/380 nm) with a Hamamatsu C9100EM-CCD camera (512 × 512 pixels) and acquired with Metafluor software (Molecular Devices). Custom-made IGOR Pro software (WaveMetrics) was used to analyze calcium data (12, 28). Recurrent motifs of calcium spikes in response to GHRH were determined as described previously (12).
For real-time qPCR experiments, GH-positive cells were isolated from the other cell types (GH-negative) by FACS (FACSAria; BD Biosciences) as described previously (57). In brief, pituitary glands were dissected from 70-d-old male GH-eGFP mice (n = 10), and the neurohypophysis and intermediate lobe were removed. Cells were dissociated enzymatically by trypsin (0.5%) and mechanically by repeated gentle trituration using Pasteur pipettes. Three independent experiments were performed.
Total RNA was extracted from GH-positive and GH-negative cells and treated with DNase I using the RNAeasy Extraction Kit (Qiagen). RNA was reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen). qPCR analyses of GHRH-R and 18sRNA, used as the internal standard, were performed using SYBR Green PCR Master Mix (Applied Biosystems) as described previously (57). Primers for GHRH-R and 18sRNA were as follows: GHRH-R forward, 5′-ACCCGTATCCTCTGCTTGCT-3′; GHRH-R reverse, 5′-AGGTGTTGTTGGTCCCCTCT-3′; 18sRNA forward, 5′-GTAACCCGTTGAACCCCATT-3′; 18sRNA reverse, 5′-CCATCCAATCGGTAGTAGCG-3′. Expression of GHRH-R was normalized to the expression level of 18sRNA, according to the formula GHRH-R/18sRNA = (2−(Ct[GHRH-R] − Ct[18sRNA])) × 1,000, where Ct is the threshold cycle.
Statistical Analysis.
The effects of age and sex on the volume/surface ratio of the GH cell network were assessed using a disproportional replication test to achieve balanced group numbers. Two-way ANOVA was then performed on the resultant means, followed by Bonferroni's post hoc test to make pairwise comparisons between males and females of the same age. Calcium recordings were subjected to empirical mode decomposition (EMD) to retrieve the baseline trend (58), allowing comparison of the control and drug application periods. Under all physiological states, a GHRH response was categorized as a 25% positive deflection from the baseline value under control conditions. EMD analyses were performed using Matlab and an open-source algorithm (http://perso.ens-lyon.fr/patrick.flandrin/emd.html), which was adapted to suit the requirements of the present study. Custom-made software was used to measure the linear correlation (Pearson's R) between all pairs of GHRH-responsive GH-eGFP cells as described previously (12). The effects of sex, gonadectomy, and hormone replacement on cell activity under basal and stimulated conditions were compared using a multiple equality of proportion test, followed by a Tukey-type post hoc test to make multiple pairwise comparisons. Cell pair correlation indexes were performed using the Pearson's R. For the eGFP secretion studies, the times to peak and maximum peak amplitude values were calculated. A nonparametric Mann–Whitney U test was then used to assess the effect of sex on both parameters. In all cases, differences between groups were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université Montpellier 1 and 2, Agence Nationale de la Recherche (Pit-Net Project), Institut Fédératif de Recherches 3, and Région Languedoc Roussillon; by core funding from the Medical Research Council (United Kingdom, U117570590); by the Irish National Biophotonics and Imaging Platform; by the Irish Government's Programme for Research in Third-Level Institutions, Cycle 4; and by Ireland's EU Structural Funds Programmes 2007–2013.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010849107/-/DCSupplemental.
References
- 1.Robinson ICAF, Hindmarsh PC. The Growth Hormone Secretory Pattern and Structural Growth. New York: Oxford Univ Press; 1999. [Google Scholar]
- 2.Legraverend C, Mode A, Wells T, Robinson I, Gustafsson JA. Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretion in normal and dwarf rats. FASEB J. 1992;6:711–718. doi: 10.1096/fasebj.6.2.1537461. [DOI] [PubMed] [Google Scholar]
- 3.Mode A, Gustafsson JA. Sex and the liver: A journey through five decades. Drug Metab Rev. 2006;38:197–207. doi: 10.1080/03602530600570057. [DOI] [PubMed] [Google Scholar]
- 4.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raisman G, Field PM. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- 6.Albertsson-Wikland K, Rosberg S, Libre E, Lundberg LO, Groth T. Growth hormone secretory rates in children as estimated by deconvolution analysis of 24-h plasma concentration profiles. Am J Physiol. 1989;257:E809–E814. doi: 10.1152/ajpendo.1989.257.6.E809. [DOI] [PubMed] [Google Scholar]
- 7.Painson JC, Thorner MO, Krieg RJ, Tannenbaum GS. Short-term adult exposure to estradiol feminizes the male pattern of spontaneous and growth hormone–releasing factor–stimulated growth hormone secretion in the rat. Endocrinology. 1992;130:511–519. doi: 10.1210/endo.130.1.1345780. [DOI] [PubMed] [Google Scholar]
- 8.Painson JC, Veldhuis JD, Tannenbaum GS. Single exposure to testosterone in adulthood rapidly induces regularity in the growth hormone release process. Am J Physiol Endocrinol Metab. 2000;278:E933–E940. doi: 10.1152/ajpendo.2000.278.5.E933. [DOI] [PubMed] [Google Scholar]
- 9.Baccam N, et al. Dual-level afferent control of growth hormone–releasing hormone (GHRH) neurons in GHRH-green fluorescent protein transgenic mice. J Neurosci. 2007;27:1631–1641. doi: 10.1523/JNEUROSCI.2693-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouyer K, Faivre-Bauman A, Robinson IC, Epelbaum J, Loudes C. Sexually dimorphic distribution of sst2A receptors on growth hormone–releasing hormone neurones in mice: modulation by gonadal steroids. J Neuroendocrinol. 2008;20:1278–1287. doi: 10.1111/j.1365-2826.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 11.Osterstock G, et al. Ghrelin stimulation of growth hormone–releasing hormone neurons is direct in the arcuate nucleus. PLoS ONE. 2010;5:e9159. doi: 10.1371/journal.pone.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnefont X, et al. Revealing the large-scale network organization of growth hormone–secreting cells. Proc Natl Acad Sci USA. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ, Bootman MD, Lipp P. Calcium: A life-and-death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 15.Magoulas C, et al. A secreted fluorescent reporter targeted to pituitary growth hormone cells in transgenic mice. Endocrinology. 2000;141:4681–4689. doi: 10.1210/endo.141.12.7828. [DOI] [PubMed] [Google Scholar]
- 16.Lafont C, et al. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA. 2010;107:4465–4470. doi: 10.1073/pnas.0902599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite E, et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology. 2010;151:234–243. doi: 10.1210/en.2009-0539. [DOI] [PubMed] [Google Scholar]
- 18.Le Tissier PR, et al. Hypothalamic growth hormone–releasing hormone (GHRH) deficiency: targeted ablation of GHRH neurons in mice using a viral ion channel transgene. Mol Endocrinol. 2005;19:1251–1262. doi: 10.1210/me.2004-0223. [DOI] [PubMed] [Google Scholar]
- 19.Jansson JO, Ekberg S, Isaksson OG, Edén S. Influence of gonadal steroids on age- and sex-related secretory patterns of growth hormone in the rat. Endocrinology. 1984;114:1287–1294. doi: 10.1210/endo-114-4-1287. [DOI] [PubMed] [Google Scholar]
- 20.Jansson JO, Ekberg S, Isaksson O, Mode A, Gustafsson JA. Imprinting of growth hormone secretion, body growth, and hepatic steroid metabolism by neonatal testosterone. Endocrinology. 1985;117:1881–1889. doi: 10.1210/endo-117-5-1881. [DOI] [PubMed] [Google Scholar]
- 21.Jansson JO, Frohman LA. Differential effects of neonatal and adult androgen exposure on the growth hormone secretory pattern in male rats. Endocrinology. 1987;120:1551–1557. doi: 10.1210/endo-120-4-1551. [DOI] [PubMed] [Google Scholar]
- 22.Hoeffler JP, Frawley LS. Capacity of individual somatotropes to release growth hormone varies according to sex: Analysis by reverse hemolytic plaque assay. Endocrinology. 1986;119:1037–1041. doi: 10.1210/endo-119-3-1037. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel W, et al. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329:719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- 24.Guérineau NC, Bonnefont X, Stoeckel L, Mollard P. Synchronized spontaneous Ca2+ transients in acute anterior pituitary slices. J Biol Chem. 1998;273:10389–10395. doi: 10.1074/jbc.273.17.10389. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AA, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322:1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- 26.Brazeau P, et al. Somatocrinin (growth hormone releasing factor) in vitro bioactivity: Ca++ involvement, cAMP-mediated action and additivity of effect with PGE2. Biochem Biophys Res Commun. 1982;109:588–594. doi: 10.1016/0006-291x(82)91762-4. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Suzuki M. The time course and extracellular Ca2+ involvement of growth hormone (GH) releasing factor–induced GH secretion in perifused dispersed rat pituitary cells. Jpn J Physiol. 1986;36:1225–1239. doi: 10.2170/jjphysiol.36.1225. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont X, Fiekers J, Creff A, Mollard P. Rhythmic bursts of calcium transients in acute anterior pituitary slices. Endocrinology. 2000;141:868–875. doi: 10.1210/endo.141.3.7363. [DOI] [PubMed] [Google Scholar]
- 29.Lim NF, Nowycky MC, Bookman RJ. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990;344:449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- 30.Mollard P, Seward EP, Nowycky MC. Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization. Proc Natl Acad Sci USA. 1995;92:3065–3069. doi: 10.1073/pnas.92.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedej S, Tsujimoto T, Zorec R, Rupnik M. Voltage-activated Ca(2+) channels and their role in the endocrine function of the pituitary gland in newborn and adult mice. J Physiol. 2004;555:769–782. doi: 10.1113/jphysiol.2003.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takei T, Yasufuku-Takano J, Takano K, Fujita T, Yamashita N. Effect of Ca2+ and cAMP on capacitance-measured hormone secretion in human GH-secreting adenoma cells. Am J Physiol. 1998;275:E649–E654. doi: 10.1152/ajpendo.1998.275.4.E649. [DOI] [PubMed] [Google Scholar]
- 33.Gromada J, et al. Nateglinide, but not repaglinide, stimulates growth hormone release in rat pituitary cells by inhibition of K channels and stimulation of cyclic AMP–dependent exocytosis. Eur J Endocrinol. 2002;147:133–142. doi: 10.1530/eje.0.1470133. [DOI] [PubMed] [Google Scholar]
- 34.Sedej S, Rose T, Rupnik M. cAMP increases Ca2+-dependent exocytosis through both PKA and Epac2 in mouse melanotrophs from pituitary tissue slices. J Physiol. 2005;567:799–813. doi: 10.1113/jphysiol.2005.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashall L, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB–dependent transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SC, et al. Molecular basis of the little mouse phenotype and implications for cell type–specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 37.Bilezikjian LM, Vale WW. Stimulation of adenosine 3′,5′-monophosphate production by growth hormone–releasing factor and its inhibition by somatostatin in anterior pituitary cells in vitro. Endocrinology. 1983;113:1726–1731. doi: 10.1210/endo-113-5-1726. [DOI] [PubMed] [Google Scholar]
- 38.Painson JC, Tannenbaum GS. Sexual dimorphism of somatostatin and growth hormone–releasing factor signaling in the control of pulsatile growth hormone secretion in the rat. Endocrinology. 1991;128:2858–2866. doi: 10.1210/endo-128-6-2858. [DOI] [PubMed] [Google Scholar]
- 39.Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17:675–683. doi: 10.1016/j.conb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehrenberg WB, Giustina A. Basic counterpoint: Mechanisms and pathways of gonadal steroid modulation of growth hormone secretion. Endocr Rev. 1992;13:299–308. doi: 10.1210/edrv-13-2-299. [DOI] [PubMed] [Google Scholar]
- 42.Zhu YS, Dellovade T, Pfaff DW. Gender-specific induction of pituitary RNA by estrogen and its modification by thyroid hormone. J Neuroendocrinol. 1997;9:395–403. doi: 10.1046/j.1365-2826.1997.00590.x. [DOI] [PubMed] [Google Scholar]
- 43.Beyer C, Kolbinger W, Froehlich U, Pilgrim C, Reisert I. Sex differences of hypothalamic prolactin cells develop independently of the presence of sex steroids. Brain Res. 1992;593:253–256. doi: 10.1016/0006-8993(92)91315-6. [DOI] [PubMed] [Google Scholar]
- 44.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Portman DS. Neural sex modifies the function of a C. elegans sensory circuit. Curr Biol. 2007;17:1858–1863. doi: 10.1016/j.cub.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Billeter JC, et al. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J Physiol Sci. 2008;58:213–220. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- 49.Jansson JO, Edén S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 50.Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- 51.Harris GW, Levine S. Sexual differentiation of the brain and its experimental control. J Physiol. 1965;181:379–400. doi: 10.1113/jphysiol.1965.sp007768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 53.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 54.Martínez AS, et al. Estrogen priming effect on growth hormone (GH) provocative test: A useful tool for the diagnosis of GH deficiency. J Clin Endocrinol Metab. 2000;85:4168–4172. doi: 10.1210/jcem.85.11.6928. [DOI] [PubMed] [Google Scholar]
- 55.Yamane T, et al. Roles of neonatal and prepubertal testicular androgens on androgen-induced proliferative response of seminal vesicle cells in adult mice. J Steroid Biochem. 1987;28:559–564. doi: 10.1016/0022-4731(87)90515-2. [DOI] [PubMed] [Google Scholar]
- 56.McMillan J, et al. Osteoinductivity of demineralized bone matrix in immunocompromised mice and rats is decreased by ovariectomy and restored by estrogen replacement. Bone. 2007;40:111–121. doi: 10.1016/j.bone.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Chauvet N, et al. Characterization of adherens junction protein expression and localization in pituitary cell networks. J Endocrinol. 2009;202:375–387. doi: 10.1677/JOE-09-0153. [DOI] [PubMed] [Google Scholar]
- 58.Huang N, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond. 1998;454:903–995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.