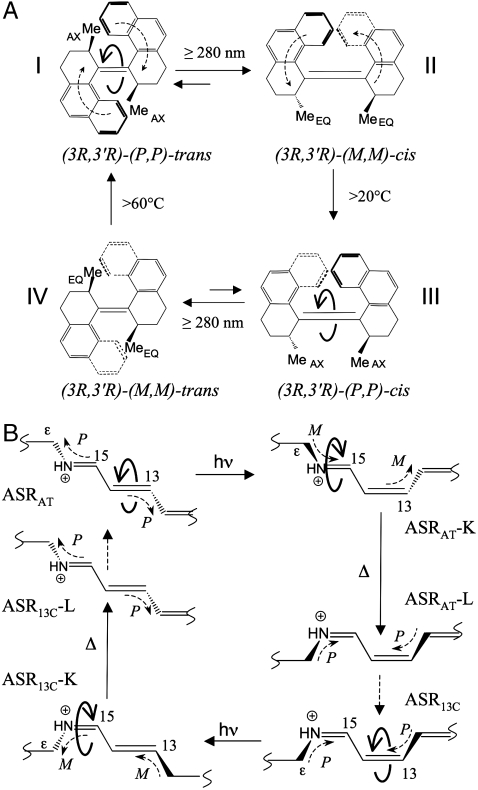
Fig. 1.
(A) The biphenanthrylidene molecular rotor fabricated by Feringa and co-workers (2). Full curly arrows indicate CCW (stations I and III) double bond isomerization. The right-handed (M) and left-handed (P) helicities (see the dashed curly arrows in stations I and II) result from steric clashing of the double bond substituents and the 3R and 3′R stereogenic centers. The II → III and IV → I thermal steps are driven by 11 and 9 kcal mol-1 stabilization of the III and I structures, respectively. (B) The photochromic cycle of ASR. Full curly arrows indicate alternating CCW and CW isomerizations about the ─C13═C14─ and ─C15═N─ retinal double bonds, respectively. The helicities of the ─C15═NH─Cε─ and ─C12─C13═C14─ moieties (dashed curly arrows) are assigned by determining whether the helicity of the corresponding ─H(C14)─C15═NH─Cε─ and ─C12─C13═C14─(C15)H─ fragments is M or P.