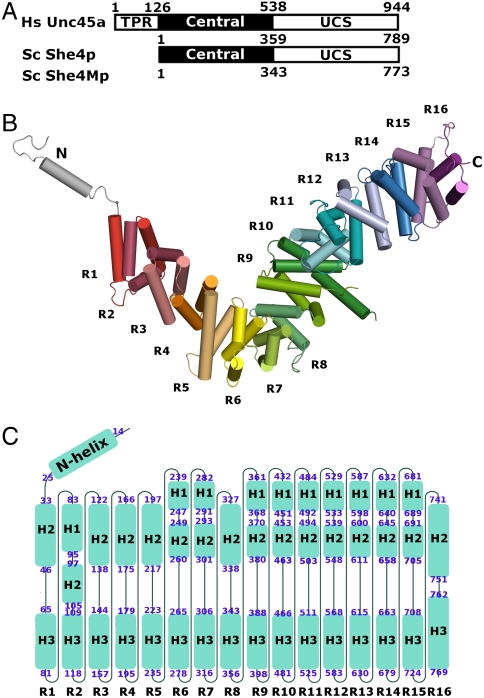
Fig. 1.
Yeast UCS (She4p) folds into helical repeats. (A) Domain organization of UCS proteins: Human Unc45a, yeast She4p, and yeast She4Mp; residue numbers at domain boundaries are indicated. All UCS proteins share central and UCS domains. Metazoan UCS proteins, exemplified here by human Unc45a, contain an additional tetratricopeptide repeat (TPR). (B) She4p assembles into helical repeats, which are shown in different colors, and are numbered from R1–R16. The repeats form an L-shaped superhelix. Extending from the short arm of the L-shaped superhelix is an N-terminal loop-helix-loop motif (silver). (C) The topology of She4p. Sixteen helical turns are arranged from left to right and labeled as R1–R16; the helices of each turn are shown as blue boxes and named according to their position in the conventional three-helical repeats as H1–H3 and in the two-helical repeat as H2–H3. The beginning and the ending residue of each helix are labeled at the ends of each box. H3 helices from 16 repeats align to form a continuous inner surface. Helix H1 of R2 and the H2 helices of the other repeats align to form a continuous outer surface in the 3D structure.