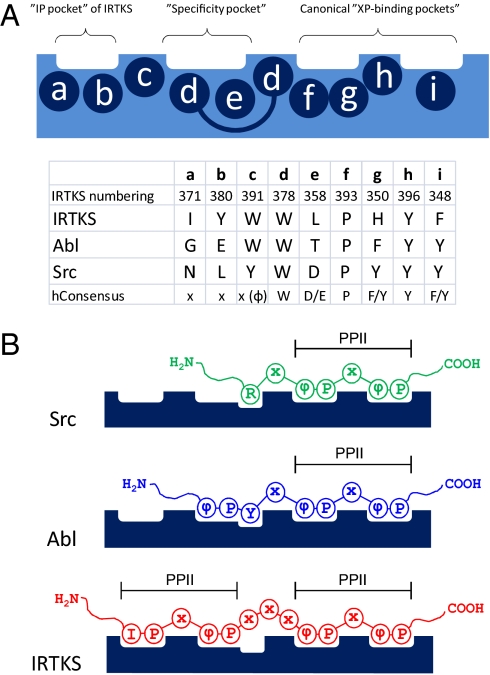
Fig. 5.
Schematic presentation of class I peptide binding to Src-, Abl-, and IRTKS-type SH3 domains. (A) Amino acids (a to i) forming the individual peptide-binding pockets of SH3 domains (numbering according to IRTKS SH3 in Fig. 1). (B) Peptide recognition is similar for all SH3 domains at the canonical “XP-binding pockets,” wherein each peptide adopts a left-handed PPII helical conformation. Selectivity is determined at the “specificity pocket,” wherein peptide conformation and peptide/SH3 domain interactions are different in each case. In IRTKS SH3, the specificity and “IP pockets” also accommodate PPII helical moiety.