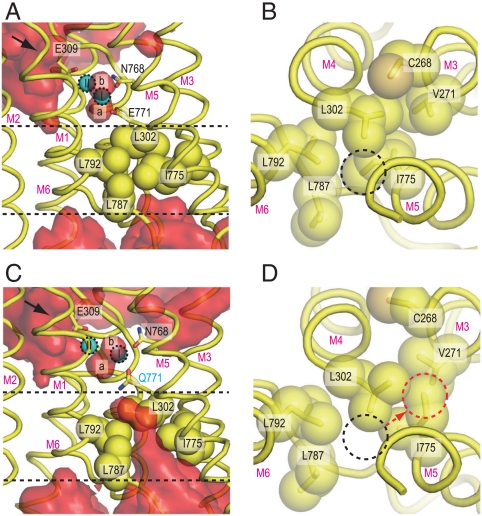
Fig. 3.
Hydrophobic shielding on the lumenal side. Atomic models in simulations for wild-type [WT1 at 10 ns; (A and B)] and for the Glu771Gln mutant [E771Q3 at 10 ns; (C and D)] are shown. (A and C) Views parallel to the membrane, and (B and D) those from the Ca2+-binding site toward the lumen. Hydrophobic residues, located between the two dashed lines in A and C, are shown in space fill (B and D). Red surfaces [(A) WT1 and (C) E771Q3] represent average water densities calculated with Maptools 1.0 (http://www.mpibpc.mpg.de/groups/de_groot/maptools.html) and contoured at 1.5σ using Pymol (33). The arrows in A and C indicate water paths leading to Glu309. The dashed circles in B and D specify the positions of the Ile775 Cδ in WT1 (black) and E771Q3 (red). Two bound Ca2+ (I and II) and water molecules (a and b) are marked.