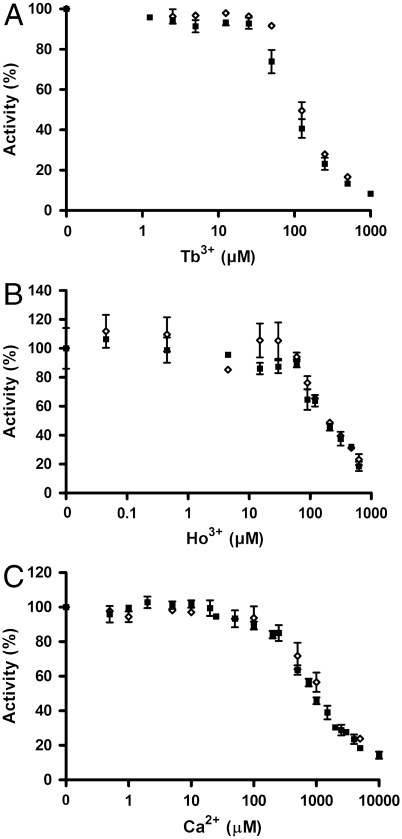
Fig. 2.
Ca2+, Tb3+ and Ho3+ ions inhibit the activity of the purified PM H+-ATPase independently of an intact autoinhibitory domain. ATP hydrolytic activity of the C-terminal truncated PM H+-ATPase (aha2Δ73) and the full-length PM H+-ATPase (AHA2) was measured with varying concentrations of: (A) Tb3+ ions, (B) Ho3+ ions, and (C) Ca2+ ions. The activity without the addition of ions were set to 100% in A, B, and C. ▪, aha2Δ73; ◊, AHA2. All experiments are represented as ± S.D.