Table 1.
Substrate scope for three-component coupling.
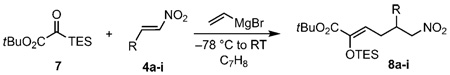 | ||
|---|---|---|
| Product[a] | R | Yield [%] |
| 8a | Ph | 72[b] |
| 8b | 4-Cl-C6H4 | 70[b] |
| 8c | 2-thienyl | 65[b] |
| 8d | 2-furyl | 64[b] |
| 8e | (E)-CH=CH-C6H5 | 66[b] |
| 8 f | H | 57[c] |
| 8g | n-pentyl | 58[b] |
| 8h | iPr | 53[c] |
| 8i | tBu | 36[c] |
Reagents: Silyl glyoxylate (1.5 equiv), nitroalkene (1 equiv), CH2= CHMgBr (1.5 equiv), [4]0=0.1m in C7H8;
Yield of isolated product.
Yields were determined by 1H NMR spectroscopy of crude product compared to an internal standard of mesitylene.
