Figure 5. J-protein function with or without client binding.
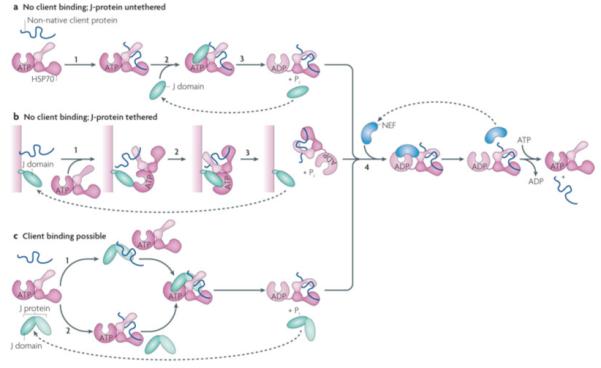
J-proteins can act without binding to clients, either untethered (a) or tethered to a particular site in the cell (b). In the case of J-proteins having client protein binding domains, clients may bind either bind the J-protein before interaction with Hsp70 or only serve to stimulate Hsp70’s ATPase activity (c) a| The simplest J-protein function is the action of a J-domain in the absence of a client protein binding domain to stimulate the ATPase activity, allowing it to capture a client protein that has transiently entered the open peptide-binding cleft of Hsp70(ATP) (i), by binding Hsp70 (ii) and stimulating ATP hydrolysis (iii). In such cases, Hsp70 is the driving force of client protein interaction, as there is no facilitation by the J-protein, either through direct binding or by subcellular localization. The in vivo function of a J-domain lacking other sequences has been demonstrated experimentally44. b|J-domains either lacking (shown) or having (not shown) client protein binding domains are often tethered to a site rich in client proteins. In this way, upon initiation of client protein binding by Hsp70(ATP) (1), a high concentration of J-domains is present (2) facilitating ATP hydrolysis and thus client capturing by Hsp70 (3). c|In the case of J-proteins having client protein binding domains, two modes of J-protein function can occur: top: as in the canonical model of J-protein:Hsp70 function J-protein binds client first (x) and targets it to Hsp70 (y,z); bottom: binding occurs directly to Hsp70 (x’) as described in a); in such cases J-proteins stimulate Hsp70 ATPase activity only even though a client binding domain is present (y’,z). Evidence for such an alternative pathway has been found in the mitochondrial Fe-S cluster biogenesis pathway in yeast with the specialized J-protein Jac1 and Hsp70 Ssq1142. In all cases, release of client is facilitated by NEFs (iv).
