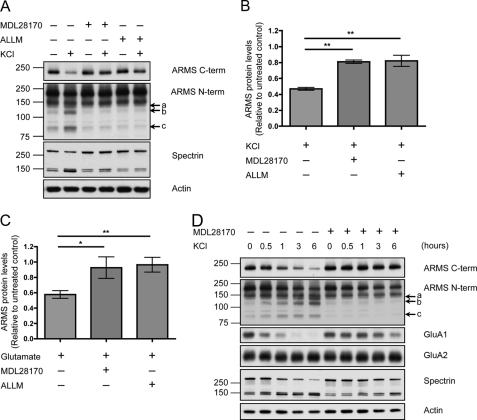
FIGURE 6.
ARMS/Kidins220 is degraded by calpain upon neuronal activation. A, inhibition of the protease calpain rescues KCl-induced degradation of ARMS/Kidins220. Hippocampal cultures were depolarized with 50 mm KCl for 3 h in the presence of the calpain inhibitor MDL28170 or N-acetyl-Leu-Leu-Met (ALLM), and ARMS/Kidins220 protein levels were assessed by Western blot. Both of these inhibitors significantly reduced the degradation of ARMS/Kidins220 and prevented the appearance of N-terminal degradation fragments (arrows a, b, and c). The 145- and 150-kDa breakdown products of spectrin mark calpain activity, and actin is shown as a loading control. 95- and 150-kDa nonspecific bands were detected by the N-terminal antibody. B, quantification of ARMS/Kidins220 protein levels in A, as detected by the C-terminal antibody, normalized to actin levels, and plotted relative to untreated control (n ≥ three independent experiments; **, p < 0.01, t test; data are represented as the means ± S.E.). C, hippocampal cultures were stimulated with 200 μm glutamate for 1 min in the presence of the calpain inhibitor MDL28170 or N-acetyl-Leu-Leu-Met, and cell lysates were collected at the indicated times after stimulation. ARMS/Kidins220 protein levels, as detected by the C-terminal antibody, were assessed by Western blot and quantified. ARMS/Kidins220 levels were normalized to actin levels and plotted relative to untreated control (n ≥ three independent experiments; *, p < 0.05, t test; data are represented as the means ± S.E.). D, hippocampal cultures were depolarized with 50 mm KCl for the indicated times in the presence or absence of the calpain inhibitor MDL28170, and the indicated proteins were probed by Western blot. The degradation of full-length ARMS/Kidins220, as well as the appearance of N-terminal degradation fragments (arrows a, b, and c), was correlated with the degradation of GluA1, and the degradation of both proteins was prevented by calpain inhibition. In contrast, GluA2 was not degraded.