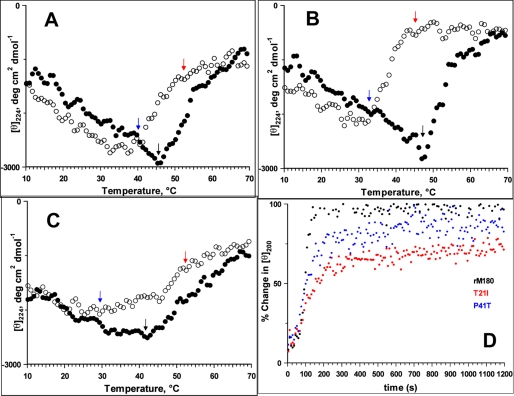
FIGURE 2.
Effects of mutations on the thermal properties of rM180. Thermal denaturation (solid circles)/refolding (open circles) and refolding kinetics of amelogenins monitored by CD spectra are shown: rM180 (A), T21I (B), and P41T (C). The arrows in the figures indicate the temperatures of onset of denaturation (black arrow), onset of refolding (red arrow), and complete refolding (blue arrow). The proteins were denatured by heating from 10 to 70 °C at 5 °C/min. Refolding was monitored between 70 and 10 °C at 5 °C/min. D, refolding kinetics of amelogenin monitored by CD ellipticity at 200 nm and 25 °C. The heat-denatured proteins were cooled to 25 °C within ∼70 s, and the change in ellipticity was monitored over time. The time-dependent changes in the CD intensity at 200 nm are normalized to the initial CD intensity (at 25 °C) before denaturation of the proteins.