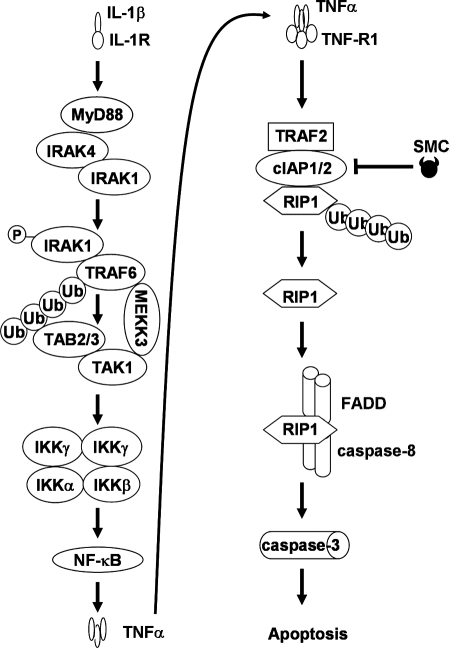
FIGURE 8.
Schematic of IL-1β-mediated SMC-induced apoptosis. Upon IL-1β occupancy, IL-1R transduces a signal by recruiting adaptor protein MyD88, which in turn recruits IRAK1 and IRAK4 (51). IRAK1 is likely phosphorylated and activated by IRAK4, allowing IRAK1 to interact with TRAF6. Autoubiquitination of TRAF6 recruits TAB2 and TAB3, and the binding of MEKK3 to TAK1 may stabilize TRAF6-associated signaling complex, allowing the cascade involving MEKK3, TAK1, and IKK complex to proceed, leading to NF-κB activation. In turn, activation of NF-κB results in production of TNFα, which is secreted to engage the TNF-R1 signaling complex in an autocrine fashion. In the presence of TRAF2, cIAP1, and cIAP2, cells respond to TNFα stimulation by promoting RIP1 ubiquitination. However, as a result of SMC treatment, cIAP1 and cIAP2 are down-regulated, thereby preventing ubiquitination of nonubiquitinated RIP1 and allowing RIP1 to bind and activate the death-inducing complex II that includes caspase-8 and FADD. Death-inducing complex II in turn activates caspase-3, promoting apoptosis.