FIGURE 3.
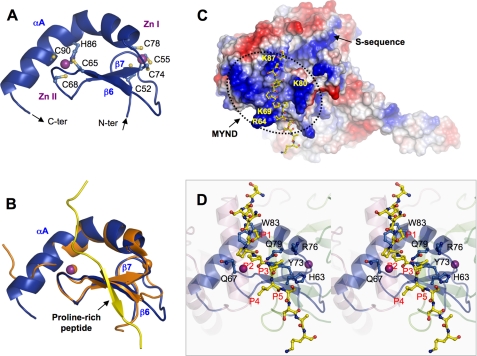
Zinc finger MYND domain. A, ribbon diagram of the MYND domain structure. Zinc ligands are labeled and represented by ball-and-stick, and zinc ions by purple spheres. The N- and C termini are indicated. B, superposition of the MYND domains of SmyD1 (blue) and AML1/ETO (PDB code 2ODD) (orange). The proline-rich peptide from AML1/ETO containing the PPPLI motif is displayed as ribbon and colored in yellow. C, surface representation of SmyD1 with coloring according to the electrostatic potential: red, white, and blue correspond to negative, neutral, and positive potentials, respectively. The surface of MYND, outlined by a dotted line, exhibits numerous positive charges due to the clustering of basic amino acids. The conserved arginine and lysine residues are labeled. The modeled proline-rich peptide, represented by ball-and-stick with carbon atoms colored in yellow, is seen to bind on the surface of SmyD1. The N terminus of the peptide projects toward the S-sequence, which appears to extend the positively charged MYND surface. D, stereo view ribbon diagram illustrates the interaction between the MYND domain and the modeled proline-rich peptide. Residues in SmyD1 that are potentially important for peptide interaction are represented by ball-and-stick with carbon atoms colored in blue. Residues in the modeled peptide are denoted according to their position in the PXLXP motif, with the 1st proline as P1 and last as P5.