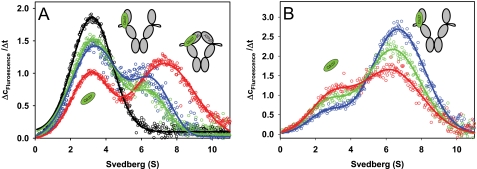
FIGURE 2.
Cdc37 binds open and closed Hsp90 conformations. A, Δc/Δt plots were generated from sedimentation velocity experiments of 150 nm *Cdc37 in standard buffer containing high salt (150 mm KCl) in the absence (black) or presence of 4 μm Hsp90 (blue). The influence of nucleotides was detected by the addition of 4 mm AMP-PNP (red) or 4 mm ADP (green) to 150 nm *Cdc37 and 4 μm Hsp90. Data fitting was performed as described under “Experimental Procedures.” B, Δc/Δt plots were generated from sedimentation velocity experiments of 150 nm *Cdc37 and 2 μm F340E Hsp90 in standard buffer containing low salt (20 mm KCl) in the absence of nucleotides (blue) or in the presence of 4 mm AMP-PNP (green) or 4 mm ATPγS (red). The schemes represent the proposed conformations of Hsp90, with *Cdc37 depicted in green.