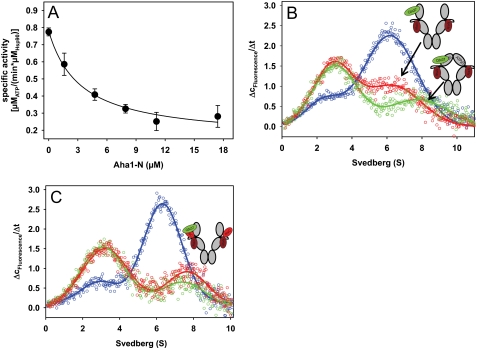
FIGURE 5.
Cdc37 displacement from the Hsp90 scaffold is dependent on the Aha1 C-domain and requires conformational changes within the Hsp90 dimer. A, the competition experiment between the N-terminal domain of Aha1 and full-length Aha1 was performed by adding increasing concentrations of Aha1-N to Hsp90 stimulated with 2 μm Aha1. Assays were performed in standard buffer containing low salt (20 mm KCl). B, Δc/Δt plots were generated from sedimentation velocity experiments of 150 nm *Cdc37 in the presence of 2 μm Hsp90 (blue). 5 μm Aha1-N was added to 150 nm *Cdc37 and 2 μm Hsp90 either in the absence (red) or in the presence of 4 mm AMP-PNP (green). Experiments were performed in standard buffer containing low salt (20 mm KCl). C, Δc/Δt plots were generated from sedimentation velocity experiments of 150 nm *Cdc37 in the presence of 2 μm F340E Hsp90 without cofactors (blue), with 5 μm Aha1 (red), or with 5 μm Aha1 and 4 mm AMP-PNP (green). The schemes represent the proposed conformations of Hsp90, with *Cdc37 depicted in green and Aha1 in red. Error bars, S.D.