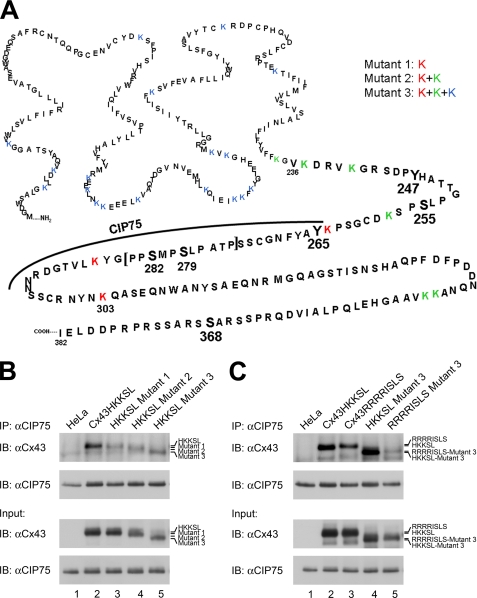
FIGURE 6.
CIP75 interacts with Cx43 that lacks lysine ubiquitination sites. A, site-directed mutagenesis was performed to mutate lysine residues to arginine to eliminate potential ubiquitination sites. Mutant 1 consists of mutations K264R, K287R, and K303R (red) because these wild-type residues appear to be involved or near those that are involved in CIP75 binding. Mutant 2 consists of the same mutations in Mutant 1 plus the additional mutations K236R, K239R, K243R, K345R, and K346R (green). Mutant 2 represents mutations of all lysine residues in the C-terminal tail. In Mutant 3, all lysine residues in Cx43 are mutated (blue). B and C, co-immunoprecipitation of transiently transfected HeLa cells. B, HeLa cells were transfected with either Cx43HKKSL, Mutant 1, Mutant 2, or Mutant 3. Untransfected HeLa cells were used as the negative control. Immunoprecipitated CIP75 interacted with Cx43HKKSL (B, lane 2), Mutant 1 (B, lane 3), Mutant 2 (B, lane 4), and Mutant 3 (B, lane 5), suggesting that ubiquitination of ER-localized Cx43 is not necessary for the interaction with CIP75. B, HeLa cells were transfected with either Cx43HKKSL, Cx43RRRRISLS, Cx43HKKSL Mutant 3, or Cx43RRRRISLS Mutant 3. CIP75 can also interact with the ER-retained wild-type Cx43RRRRISLS (C, lane 3) and Cx43RRRRISLS Mutant 3 (C, lane 5), which does not contain any lysines for ubiquitination. Input levels represent 2% of the amount of lysates used for immunoprecipitations. IP, immunoprecipitation; IB, immunoblot.