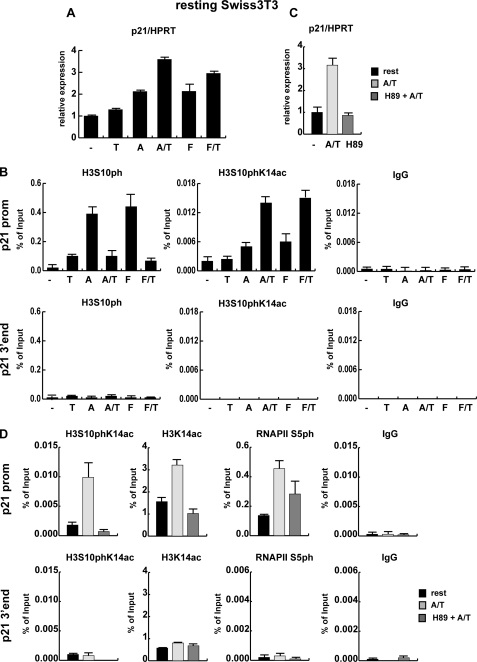
FIGURE 2.
Activated MAP kinase cascades are required for p21 expression. A, resting Swiss 3T3 mouse fibroblasts were left untreated (-) or were treated with 165.5 nm TSA alone (T), 188.5 nm anisomycin alone (A), or both drugs simultaneously (A/T) or were stimulated with 20% serum (F) either alone or in combination with 165.5 nm TSA (F/T) for 1 h. Total RNA was extracted, and reverse-transcribed cDNA was used to quantify p21 mRNA levels by qRT PCR using hypoxanthine-guanine phosphoribosyltransferase (HPRT) as a housekeeping gene for normalization. The difference in p21 expression between untreated resting cells and TSA-treated cells is not significant (p value > 0.05), whereas all other treatments resulted in significant changes in p21 expression. B, chromatin was prepared from cells treated as described in panel A and precipitated using an unspecific antibody (IgG), an histone H3S10ph antibody, and an antibody specifically recognizing histone H3 phosphoacetylated on serine 10 and lysine 14 (H3S10phK14ac). Precipitated DNA was analyzed by qRT PCR using primers specific for the promoter (upper panel) and the 3′ end (lower panel) of the p21 gene. Panels C and D, resting Swiss 3T3 mouse fibroblasts were treated for 3 h with 188.5 nm anisomycin and 165.5 nm TSA with (H89 + A/T) or without (A/T) pretreatment for 15 min with 10 μm H89. C, total RNA was extracted from stimulated cells, and reverse-transcribed cDNA was used for p21 expression analysis by qRT PCR. D, ChIP assays of resting and anisomycin/TSA stimulated 3T3 cells in the presence and absence of H89 are shown. Chromatin was precipitated with an unspecific control antibody (IgG) or antibodies specific for H3S10phK14ac, H3K14ac, H3 C terminus, and initiation-competent RNA polymerase II (RNAP II phS5), and qRT PCR was performed with primers for the p21 promoter (p21 prom) and the 3′end of the p21 gene (p21 3′ end) as control. ChIP results are shown as percentage of input; for histone modifications the values were corrected for changes in nucleosomal density.