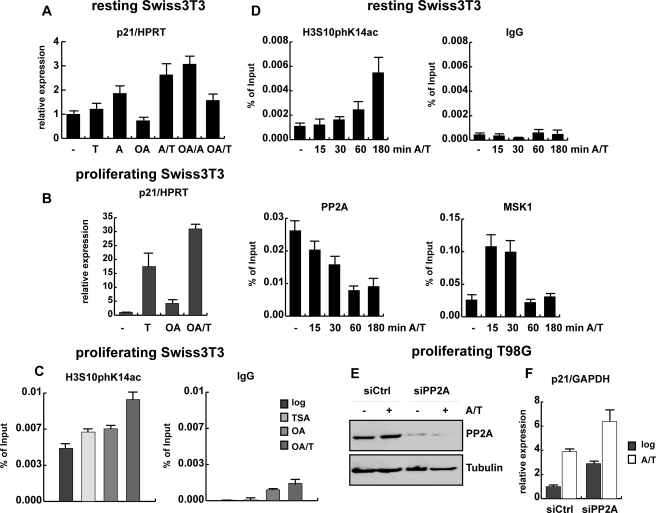
FIGURE 4.
PP2A acts a as negative regulator of p21 expression. A, resting Swiss 3T3 cells obtained by serum deprivation for 72 h were left untreated or treated for 5 h as indicated with the following concentrations: 165.5 nm TSA (T), 188.5 nm anisomycin (A), or 50 nm okadaic acid (OA) alone or in combination. Expression of p21 mRNA was examined by qRT PCR normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) and is shown relative to untreated cells. The difference in p21 expression between untreated and TSA-treated cells is not significant (p value: 0.051). B, proliferating Swiss 3T3 cells were induced with the same stimuli and for the same time period as described in panel A. Total RNA was extracted, and reverse-transcribed cDNA was used for p21 expression analysis by qRT PCR as described for panel A. C, qRT-ChIP analysis of proliferating Swiss 3T3 fibroblasts either left untreated or stimulated with 50 nm okadaic acid (OA), and 165.5 nm TSA alone or simultaneously (OA/T) using a phosphoacetylhistone H3 antibody (H3S10phK14ac) or an unspecific antibody (IgG). D, a ChIP assay of a time course is shown in which resting cells were stimulated with 188.5 nm anisomycin and 165.5 nm TSA for short time periods (indicated in minutes) using an unspecific antibody (IgG) or antibodies specific for H3S10phK14ac, the catalytic subunit of PP2A, and MSK1. ChIP results are shown as percentage of input; for histone modifications the values were corrected for changes in nucleosomal density. E and F, proliferating T98G cells were transfected with control siRNA oligonucleotides and siRNA oligonucleotides specifically targeting the two isoforms of the catalytic subunit of PP2A. Protein extracts and total RNA were gained from control (siCtrl) and knockdown cells (siPP2A) that were left untreated or were stimulated with 188.5 nm anisomycin and 165.5 nm TSA (A/T) for 1 h. E, knockdown efficiency was analyzed by Western blot using an antibody against the catalytic subunit of PP2A. An antibody specific for β-tubulin was used to ensure equal loading. F, p21 mRNA expression levels in control and PP2A knockdown cells were monitored by qRT PCR using GAPDH for normalization.