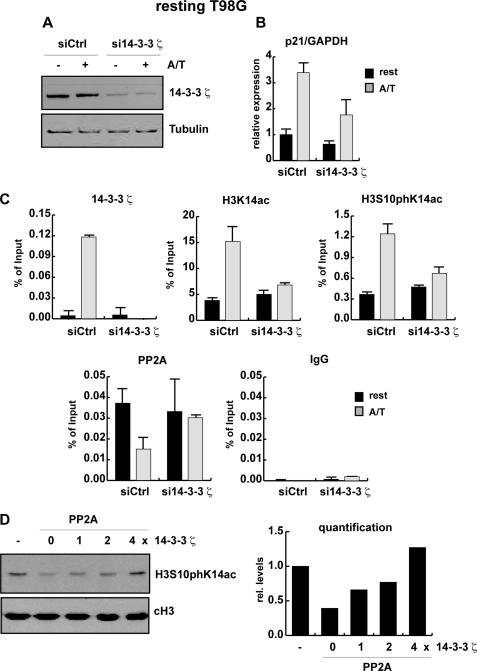
FIGURE 5.
14-3-3ζ is important for p21 activation. A–C, proliferating T98G cells were transfected with control siRNA oligonucleotides and siRNA oligonucleotides specifically targeting 14-3-3ζ mRNA. Transfected T98G cells were arrested by serum deprivation. Whole cells extracts, total RNA, and chromatin were gained from control (siCtrl) and knockdown cells (si14-3-3ζ) that were left untreated (rest) or were stimulated with 188.5 nm anisomycin and 165.5 nm TSA (A/T) for 1 h. A, knockdown efficiency was analyzed by Western blot using an antibody against 14-3-3ζ. Equal loading was controlled with an antibody specific for β-tubulin. B, p21 expression levels in control and 14-3-3ζ knockdown cells were monitored by real time PCR using GAPDH for normalization. C, shown are qRT-ChIP assays using antibodies specific for 14-3-3ζ, H3K14ac, H3S10phK14ac, the catalytic subunit of PP2A, and an unspecific antibody (IgG) as control. ChIP results are shown as percentage of input; for histone modifications, the values were corrected for changes in nucleosomal density. D, shown are an in vitro phosphorylation assay and 14-3-3 protection. Phosphoacetylated histone H3 was incubated without (-) or with recombinant PP2A in the absence (PP2A) or presence of increasing amounts of recombinant 14-3-3ζ. Phosphoacetylated histone H3 was detected with an H3S10phK14ac antibody, and equal loading was confirmed with an antibody specific for the C terminus of histone H3 (cH3). Intensities of histone H3S10phK14ac signals were quantified by densitometric scanning and normalized to the loading control (cH3).