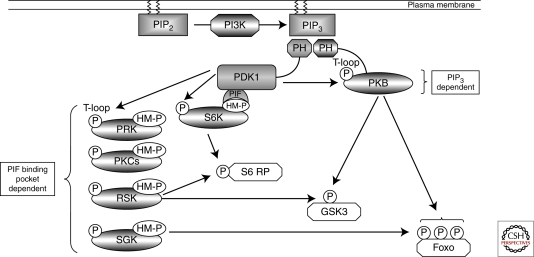
Figure 2.
PDK1 signaling. PDK1 acts as a “master kinase” activating multiple AGC kinases through phosphorylation of a conserved Threonine residue (“T-loop” site) within the catalytic domain. PDK1 activation of PKB requires the second messenger molecule PIP3 that accumulates in the plasma membrane because of the action of the lipid kinase PI3K. PDK1 activation of other AGC kinases relies on prior phosphorylation of a Ser/Thr residue within a hydrophobic motif (HM). This phosphorylated motif creates a docking site for PDK1 to bind through its PIF binding pocket, thus allowing PDK1-mediated phosphorylation of the T-loop site and kinase activation. Significant redundancy exists in PDK1 signaling as many downstream substrates, such as S6 ribosomal protein, GSK3, and FoxO transcription factors, can be phosphorylated by multiple PDK1-regulated AGC kinases.