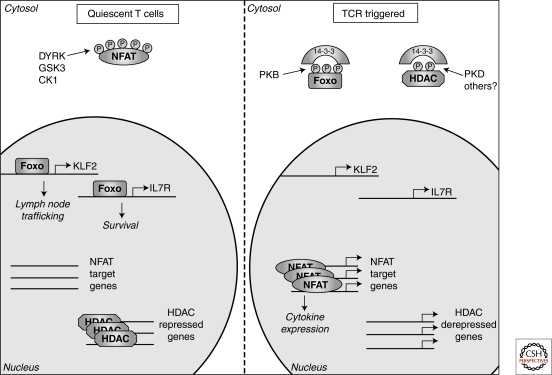
Figure 4.
Phosphorylation determined intracellular localization. In quiescent T cells constitutively active kinases (DYRK, GSK3, and CK1) maintain NFAT transcription factors in a phosphorylated state excluding them from the nucleus. In contrast, the kinases that regulate FoxO transcription factors and histone deacetylases (HDACs) are inactive in quiescent cells allowing FoxOs to access the nucleus and control genes involved in T-cell trafficking and survival whereas HDACs repress gene expression through modulating chromatin structure. Following triggering of the T-cell receptor (TCR) FoxO transcription factors and HDACs are phosphorylated, resulting in their cytosolic sequestration by 14-3-3 proteins, thus preventing their nuclear actions. Meanwhile, the Ca2+ activated phosphatase, calcineurin, dephosphorylates NFAT transcription factors allowing nuclear entry and the expression of key cytokine genes.