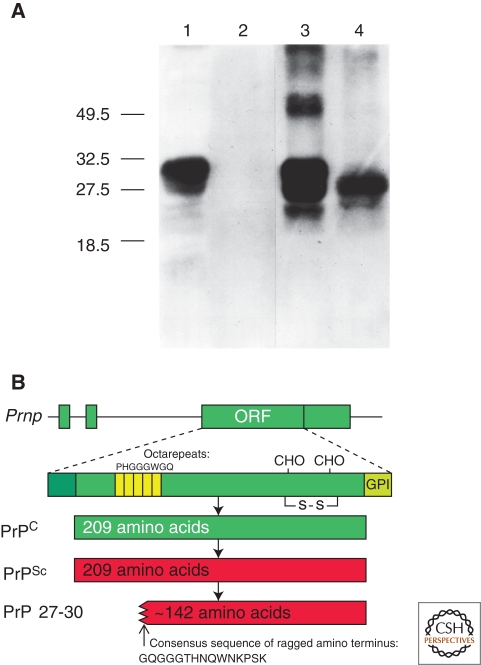
Figure 1.
Prion protein isoforms. (A) Western immunoblot of brain homogenates from uninfected (lanes 1 and 2) and prion-infected (lanes 3 and 4) Syrian hamsters. Samples in lanes 2 and 4 were digested with 50 µg/µl proteinase K for 30 min at 37°C, completely hydrolyzing PrPC. Proteinase digestion cleaves ∼67 amino acids from the amino terminus of PrPSc to generate PrP 27–30 (lane 4). Blot developed with anti-PrP polyclonal antiserum R073 (Serban et al. 1990). (B) Bar diagrams of the hamster Prnp gene and PrP isoforms. The Prnp ORF encodes a protein of 254 residues, which is shortened to 209 residues during posttranslational processing. PrPSc is an alternate conformation of PrPC with identical primary structure. Limited proteolysis of PrPSc cleaves the amino terminus and produces PrP 27-30, composed of approximately 142 residues. Panel A, reprinted with permission, from Prusiner 2004.