Abstract
Objectives:
Accurate laparoscopic liver lesion targeting for biopsy or ablation depends on the ability to merge laparoscopic and ultrasound images with proprioceptive instrument positioning, a skill that can be acquired only through extensive experience. The aim of this study was to determine whether using magnetic positional tracking to provide three-dimensional, real-time guidance improves accuracy during laparoscopic needle placement.
Methods:
Magnetic sensors were embedded into a needle and laparoscopic ultrasound transducer. These sensors interrupted the magnetic fields produced by an electromagnetic field generator, allowing for real-time, 3-D guidance on a stereoscopic monitor. Targets measuring 5 mm were embedded 3–5 cm deep in agar and placed inside a laparoscopic trainer box. Two novices (a college student and an intern) and two experts (hepatopancreatobiliary surgeons) targeted the lesions out of the ultrasound plane using either traditional or 3-D guidance.
Results:
Each subject targeted 22 lesions, 11 with traditional and 11 with the novel guidance (n = 88). Hit rates of 32% (14/44) and 100% (44/44) were observed with the traditional approach and the 3-D magnetic guidance approach, respectively. The novices were essentially unable to hit the targets using the traditional approach, but did not miss using the novel system. The hit rate of experts improved from 59% (13/22) to 100% (22/22) (P < 0.0001).
Conclusions:
The novel magnetic 3-D laparoscopic ultrasound guidance results in perfect targeting of 5-mm lesions, even by surgical novices.
Keywords: lesion targeting, ultrasound, laparoscopic, image guidance
Introduction
The targeting of structures or lesions for biopsies, catheter placement or ablation is common in many fields of medicine and surgery, particularly in liver surgery. Without the guidance of imaging modalities, the surgeon must rely on expert knowledge of anatomy in combination with tactile sense to successfully identify the location of a structure and the subsequent placement of a needle for biopsy or ablation.1 Many structures are not susceptible to palpation because they are located deep within the liver. Guessing the most likely location of a structure based on experience and anatomical landmarks is the only alternative in the absence of real-time guidance. The most accessible image guidance is ultrasound. Ultrasound has revolutionized image-guided targeting and allowed many procedures to be performed in regular medical clinics or at the bedside. However, the ultrasound operator must bear the cognitive load of correlating features in the ultrasound image to their corresponding locations in the anatomy prior to advancing a needle into tissue. This is a relatively difficult task and extensive experience is required to master the technique.2,3 Even in the best hands, the potential for error associated with ultrasound-guided needle placement is recognized as an obstacle to perfect clinical outcomes and as increasing the risk for pain and/or injury to the patient and the potential for incomplete ablations and tumour recurrence.1,4–7
An ideal guidance system takes the guesswork out of the equation by combining the imaging of the target with the exact location of the instrument(s) such that a course can be plotted and followed into the target. This information must be presented to the operator in such a way that makes the complex three-dimensional relationships between the tissue, needle, needle trajectory and transducer obvious,8–10 and minimizes the surgeon's mental workload. All of this must occur instantly and continuously.
Our efforts in developing such technology have resulted in the evolution of a system (K083728) which has been approved by the US Food and Drug Administration (FDA). It is based on dual-camera triangulation of infrared light reflected from specialized reflectors,11,12 which are attached to the ultrasound probe and needle. Using these data, the spatial relationships between the ultrasound image and the instruments can be calculated. The system thus presents a virtual representation of the needle and its projected course, in spatial combination with the actual ultrasound image; the user observes the placement of the needle on a stereoscopic monitor (Fig. 1). The combined components of this system allow for exceptionally accurate lesion targeting. This technology has been introduced clinically and a pilot human trial of the system in liver tumour ablation is underway.
Figure 1.

The guidance system based on optical infrared tracking. The user is holding a needle and ultrasound transducer, onto which are clipped tracking beacons. The motion of these beacons is observed by the infrared cameras (located within the white bar at top right), and their positions are triangulated. The guidance software represents the instruments in their actual spatial relationship, in real time, on the ‘3-D’ monitor, which is viewed by the user through stereoscopic glasses
Although the current system is effective in achieving high accuracy with little or no experience, it requires the maintenance of a direct line of sight between the tracked instruments and the infrared cameras (Fig. 2). Laparoscopy represents the ultimate challenge in this respect because the ultrasound location and the direct view of the tissue and needle insertion location are obscured by the skin. Reflectors may be attached to rigid versions of the laparoscopic instruments and ultrasound; however, preliminary work in our laboratory has demonstrated feasibility but diminished applicability of this reflection-based system as a result of significant issues regarding precision in relation to the altering of viewing angles and torque on the instruments.
Figure 2.
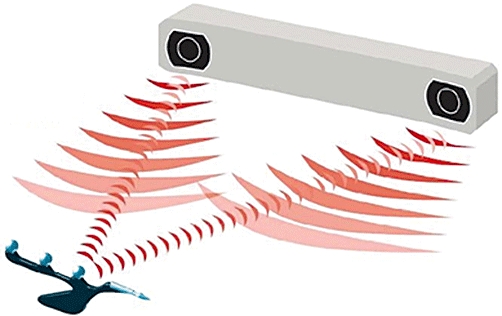
The optical tracking system requires a line of sight to the tracking beacons on the instruments it is tracking
The ideal positioning device would not be compromised by the lack of an optical line of sight, even when the cameras cannot see through the skin. One candidate technology is based on electromagnetic tracking in which the position and orientation of magnetic sensors are demonstrated within a magnetic field emanating from a field generator.13,14 The sensors' positions can be determined by measuring changes in the strength of the magnetic field, even when objects are located between the sensor and the field generator.15,16 If it can be attached to the ultrasound probe and needle during laparoscopy, this technology might overcome all previously noted problems. We hypothesized that magnetic field position guidance and 3-D virtual reality representation of the instruments might allow for the development of a system with a high degree of precision and accuracy in a model of laparoscopic, real-time, ultrasound-guided lesion targeting.
Materials and methods
Magnetic system
The guidance system operates by recording the 3-D position and orientation of the laparoscopic ultrasound transducer (LUT) and ablation needle via a magnetic motion tracker (Aurora Measurement System; Northern Digital, Inc., Waterloo, ON, Canada). The Aurora consists of a magnetic field generator, control electronics and small sensors that are installed at known locations on the instruments (Fig. 3). The controller instructs the field generator to emit a magnetic field of a known strength, based on an alternating current, at 45 Hz. When the sensors are placed inside this field, unique voltages are induced within them. The waveforms (of these voltages) are then employed to calculate the position and orientation of the sensor. As the magnetic fields are of low field strength and can safely pass through human tissue, location measurement of an object is possible within an insufflated abdomen without the demand for a line of sight imposed by an optical spatial measurement system. In our guidance system, these sensors are embedded inside the head of the flexible LUT (Fig. 4), as well as inside the tip of the ablation needle.
Figure 3.

Book-sized magnetic field generator (left). This generator was placed next to the box trainer. The sensors were connected to the registration unit (right) that interfaced with the guidance computer
Figure 4.
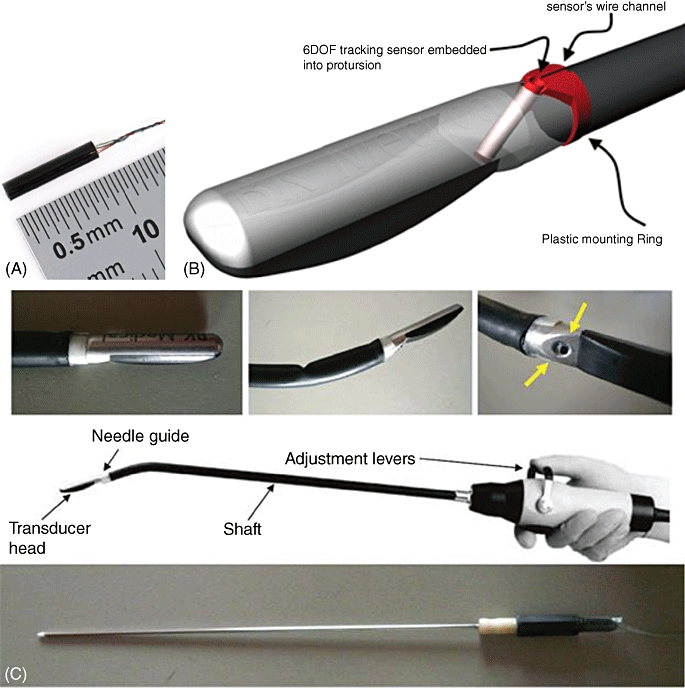
(A) A directional magnetic field sensor (0.9 cm) was attached to a moulded clip and subsequently (B) inserted into the needle guide of the laparoscopic ultrasound probe. Similarly, a sensor was inserted into a model of an ablation antenna. The actual instruments used in the experiments are not in any way hindered by the presence of the sensors. (C) The laparoscopic ultrasound retains its usual degrees of freedom
The positional information is then relayed to the guidance system software, which is equipped with computer graphics models of the instruments. The positions and orientations of these models are updated to match those of the actual instruments and are drawn on the screen to graphically represent the ultrasound slice and the needle as they are actually situated in space, in real time (Fig. 5). The guidance system's monitor is stereoscopic (3-D, similar to IMAX movie theatres), so the impact on the user is proprioceptive as well as visual. In this way, the operator can easily understand the physical relationship between the slice and the needle, as well as other salient details about the procedure. In addition to the 3-D display, the ultrasound scan is displayed in a ‘picture-in-picture’ manner in the upper left corner of the guidance system's screen.
Figure 5.
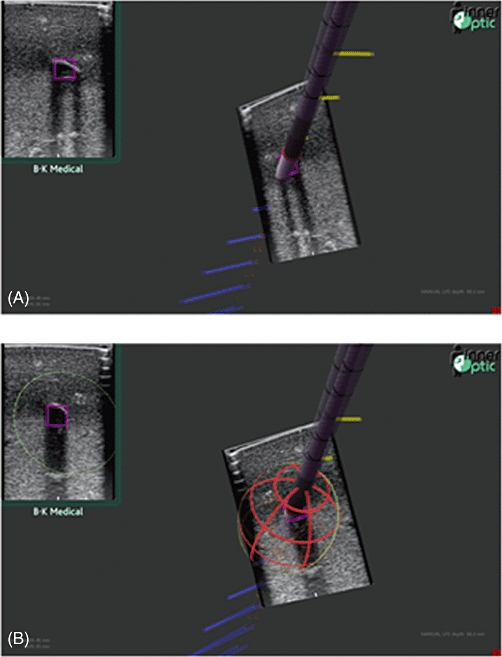
(A) Screenshots of the 3-D guidance system in use. (B) A 3-D wire cage estimates the ablation size based on previously established measures
The system indicates to the surgeon where the needle intersects the ultrasound slice, or where it would intersect the slice if advanced forward, via a pink box in the ultrasound image. This allows the surgeon to aim the needle at a target within the ultrasound slice before inserting it into the patient's anatomy because the trajectory of the needle is known. Additionally, the volume of ablated tissue can be predicted via a red–orange wire frame (Fig. 5B), which represents an energy field of a size specified by the surgeon. By this means the surgeon can assure him or herself that all tissue designated for destruction will be ablated and that no tissue or structures not intended for ablation (such as blood vessels) are within the field. The guidance system was developed with support from the Computer Science Department at the University of North Carolina at Chapel Hill over the past two decades, with our clinical involvement since 2006. The guidance system is currently in continued development in conjunction with InnerOptic Technology, Inc. (Hillsborough, NC, USA). This is not yet a commercial product.
This study also used a ProFocus 2202 ultrasound scanner from B-K Medical, Inc. (Peabody, MA, USA), with a flexible laparoscopic transducer (model #8666-RF) and a 10-mm laparoscopic endoscope, camera and light system.
Study design
Studies were performed using a custom-made laparoscopic trainer box. Ports for the laparoscopic camera and LUT were placed through the phantom trainer's top face, which consisted of a 2-cm-thick white foam plate intended to simulate the rigidity of the abdominal wall (Fig. 6). Trocars measuring 12 mm were inserted through the ports to accommodate the laparoscopic transducer and camera. The front face of the box was removed to allow the easy insertion and removal of agar-based phantoms and then covered during the experiments. The scanning depth of the ultrasound transducer was set to 5.2 cm.
Figure 6.
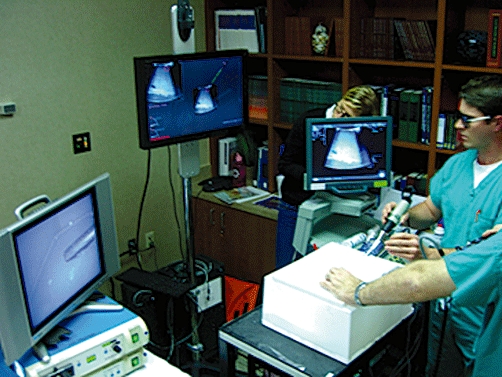
The physical layout of the experiment. The laparoscopic camera and ultrasound transducer are inserted through trocars into the white trainer box. The camera's video is viewed on the monitor (left) and the ultrasound video is viewed on the monitor (right). The central monitor provides the guidance software, in ‘3-D’ as viewed through the stereoscopic glasses seen on the participant on the right
The operators were required to attempt to pierce targets within a series of phantoms represented by agar blocks. Each agar block measured 30 × 20 × 15 cm (approximately the size of a human liver) and contained 10–12 targets (i.e. 5-mm3 pieces of egg white) at depths of either 3 cm or 5 cm. The agar phantom was released from the mould and placed upside down in the bottom of the box. The top of the agar was coated in ultrasound gel and a new phantom lesion target was chosen for each experiment. Repeat targeting was not allowed.
Using this approach, the following experimental variables were studied: (i) approach of the needle to the ultrasound slice (traditional in-plane vs. guided out-of-plane), and (ii) experience level of the subject (‘novice’ [no previous experience in ultrasound use and interpretation] and ‘expert’ [≥5 years of experience]). The novices were given a single demonstration by the experimenter of how to insert the transducer through the trocar and how to operate the levers of its flexible head prior to beginning the experiment. It was determined that the study would require the participation of four subjects (two experts and two novices) to achieve statistical significance. Each subject made 11 attempts in each condition of approach (in-plane and guided) for a total of 22 attempts per subject and 88 attempts for all conditions across all users.
The experimenter chose the target within the agar block and inserted the needle through the foam cover of the phantom trainer at any point on its surface. Subjects were allowed to select any angle of approach to ultrasound scan (i.e. ‘in-plane’ or ‘out-of-plane’). Once the needle had breached the phantom's surface, the subject was not allowed to alter its course but was instructed only to advance the needle linearly. This approach, as opposed to allowing the operator to change the trajectory following initial antenna insertion, was used to maximize the sensitivity to identify increases in accuracy and precision. In order for the attempt to qualify as a ‘hit’, the tip was required to penetrate the target. If the tip completely missed the target or if the tip of the antenna passed through the target, the attempt was considered to constitute a ‘miss’.
Before each condition, the experimenter randomly drew a slip of paper to determine under which conditions the attempt would be made. After needle insertion, the subject held the needle still and the experimenter scanned the target with a regular t-probe. Two snapshot images were generated: ‘in-plane’ and perpendicular. The snapshots were saved and designated with the number of the attempt for independent verification. After the experiments, an independent evaluator, who was blind to condition and subject, determined whether or not the attempt represented a hit.
Statistical analysis
Results were expressed as simple counts and percentages. Groups were compared using chi-squared analysis. A P-value of <0.05 was considered statistically significant. jmp Version 8 (SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis.
Results
Four subjects were tested. A total of 88 lesions were targeted. Using the traditional approach, 14 of 44 (32%) targets were hit. Using the 3-D magnetic guidance improved the hit rate to 44 of 44 (100%; P < 0.0001) (Fig. 7). Only one of 22 lesions targeted by the novice subjects was hit using the traditional approach. However, when the novice subjects used the magnetic 3-D guidance system, they hit all 22 targets (100%) (Fig. 7). By contrast, two of the four subjects were hepatopancreatobiliary (HPB) surgeons with extensive experience in using ultrasound and laparoscopy. These subjects hit 13 of 22 (59%) targets using the traditional approach and all 22 targets (100%) using the magnetic 3-D guidance system (Fig. 7).
Figure 7.
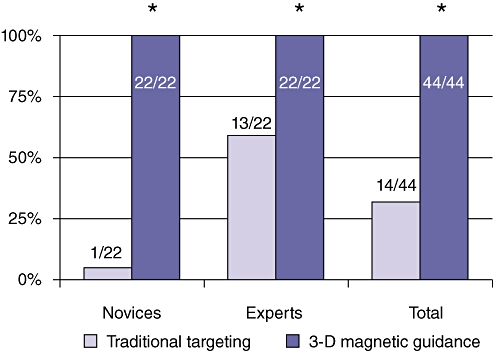
Surgical novices were essentially unable to hit any of the 5-mm targets using conventional laparoscopy and ultrasound techniques. Expert surgeons had a markedly better ability to target small lesions using traditional technology. Novices as well as expert surgeons succeeded in hitting all targets using the novel magnetic 3-D image guidance system
It is of note that the novice subjects had particular difficulty in spatially orienting the ultrasound and needle laparoscopically. They would constantly shift their attention from the laparoscopic video and ultrasound image and appeared to expend great effort on determining the correspondence between the two screens. However, when using the guidance system, they focused entirely on the 3-D display. To a lesser degree and with substantially more focus on the 2-D ultrasound images, the HPB surgeons also relied more on the direct visualization on the laparoscopic monitor during the traditional approach, compared with during the guidance system approach.
Discussion
Image guidance in medicine is of paramount importance to the avoidance or minimizing of injury and pain during needle insertion into tissue, and to improving the sensitivity of biopsy and other needle-based procedures. Achieving high rates of accuracy in the targeting of structures, even under direct ultrasound guidance, requires expert levels of training. When an additional layer of complexity is added, such as in laparoscopy, the targeting of lesions and structures becomes very difficult and is only successfully achieved by practitioners with high degrees of training, experience and expertise.1,7 Even in the hands of experts, accuracy is significantly worse than it is in open procedures. Given that increasing numbers of hepatic surgical procedures are performed laparoscopically, the importance of image guidance has become more prominent.17–20 In this study we have described a novel technology that voids all laparoscopic barriers to aid in the targeting of lesions in a liver model. Experts in laparoscopic ultrasound-guided needle placement were able to place needles into small 5-mm lesions with a reasonable degree of accuracy (59%), but the novel technology allowed them to improve this to 100%. More importantly, the technology allowed complete surgical novices to perform the same task, without any form of training or experience, also with perfect accuracy. The 3-D magnetic targeting system effectively cancelled any advantage accrued by surgical experts in years of surgical training and practice. However, we acknowledge that the current pilot study was designed from a proof-of-concept focus. A fully powered analysis with a greater number of test subjects is required to minimize potential issues with reproducibility within subjects or within subject groups.
Other imaging technologies exist to aid the surgeon in targeting structures inside the body.21,22 Computed tomography (CT) guidance systems may be able to visualize tissue with high resolution and improved grey-scale imaging, but, because of the static nature of the scans, they cannot be used in real time. As such, these systems are excellent for the planning of surgical procedures and identification of lesions. Three-dimensional modelling of the abdominal contents can facilitate understanding of lesion location in relation to other structures. Unless the subject is completely static, such as after the fixation of the skull to a targeting device during brain surgery, the absence of real-time imaging represents an insurmountable obstacle when precision and accuracy are required.23
The unique real-time properties of ultrasound allow for tissue to be scanned while procedures are underway.24–26 Although the image quality of ultrasound is inferior to that of magnetic resonance imaging (MRI) or CT, the direct application of the ultrasound probe to the surface of the liver increases sensitivity dramatically. Currently, ultrasound allows for the detection of lesions that cannot be distinguished by CT or MRI. At present, its sensitivity and provision of real-time scanning make ultrasound the ideal tool with which HPB surgeons can attain anatomical information on vasculature and tumours in the liver during resection or ablation. However, ultrasound technology is also associated with noted drawbacks, including the narrowness of the 2-D ultrasound plane, which requires constant movement of the ultrasound probe to allow the operator to ‘build’ a 3-D mental image of the tissue structures imaged. Concomitantly, it becomes increasingly difficult to target deep structures accurately when the exact approach is not captured in the narrow ultrasound plane. Because liver anatomy is complex, the optimal approach of instruments and ablation or biopsy needles to tumours often cannot be achieved in exact alignment with the ultrasound plane. This adds to the difficulty of using the mental reconstruction of the 2-D ultrasound images, which require the surgeon to plot a mental course through this tissue to reach the tumour. These inherent difficulties mean that high degrees of experience are required to attain acceptable levels of precision and accuracy in biopsy and ablation procedures. The system presented here addresses and alleviates these difficulties. By visually representing the exact relationship between the instruments and the operator, the system significantly reduces the cognitive load borne by the surgeon when trying to guide a laparoscopic needle into a feature in an ultrasound image.
This system also represents a marked improvement over its predecessor, which used optical tracking technology. Removing the requirement for the tracker to maintain line-of-sight beacons on the tracked instruments increases the range of procedures that might benefit from this guidance system substantially.
For this study, we used the Aurora tracking system, which generates AC magnetic fields using several coils embedded inside the field generator. These fields induce currents inside the sensors, which consist of passive copper wire loops around a dielectric core. The tracker measures the current in the sensor coils to determine the field strength. The fields also induce eddy currents in nearby metal objects, and these in turn generate secondary magnetic fields which reduce the quality of the tracking. The effect of the eddy currents on the sensors depends partially on the frequency of the primary magnetic fields and the properties of the metal objects. The Aurora compensates for eddy currents by varying the frequencies of the primary fields.
An alternative to an AC tracker is a pulsed-DC tracker. Pulsed-DC trackers also use several coils to generate magnetic fields. After the field has stabilized, there are no eddy currents in nearby metal objects. At this time, active fluxgate magnetometers in the sensors (each with a powered drive coil and passive sense coil wrapped around a core) measure the strength and polarity of the magnetic field. Other researchers27 have found the AC tracker to be relatively immune to the effects of metal near the sensor. This is particularly advantageous in our application as we have embedded the sensor directly into the puncture guide of the metal head of the laparoscopic ultrasound probe. By contrast, the pulsed-DC tracker is sensitive to metal near the sensor but has better orientation accuracy. Orientation accuracy is also important in our application because when the needle is outside the patient's insufflated abdomen, it is far from the target inside the liver. Errors in orientation are magnified by this large distance. In a future study, we plan more in-depth evaluation of tracking technology under conditions typical of laparoscopic liver surgery.
The current study investigated the applicability of a magnetic tracking system and virtual reality-based guidance in a laparoscopic setting. For this purpose a plastic box trainer and Styrofoam™ cover were used. The next step is to test the system in a live animal in a real operating room (OR). There is potential for the metals in the operating table and radiated emissions from other surgical devices to interfere with the accuracy of magnetic position tracking. However, several other groups have tested magnetic tracking in ORs13–16 and thus we expect these problems to be minimal and manageable. The materials used in this study are not substitutes for human tissue. Predictive modelling does not imply significant alterations of the magnetic field properties, nor would it predict significantly diminished accuracy as a result of traversing the live tissue barrier. However, this remains to be confirmed experimentally. Furthermore, the absence of metal distracters such as operating tables and retractors may potentially influence the magnetic field and subsequently the accuracy and precision of this system. Although the actual influence of OR tables and other equipment remains to be tested experimentally, theoretically at least the impact should be minimal.
The increased accuracy to be gained using the magnetic field image guidance system would be of crucial importance during laparoscopic liver surgery. More and more procedures are being pushed into the arena of minimally invasive surgery, and less invasive procedures such as laparoscopic ablation and laparoscopic biopsies largely rely on accuracy and precision for their success. Because of the complexity and variability of liver anatomy, it is only in recent decades that liver surgery has become possible. Real-time image guidance has made liver surgery substantially safer and has dramatically reduced the associated complication profile in recent years. Laparoscopic liver surgery represents the next frontier and image guidance is even more important in this field of endeavour as 3-D visual and tactile feedback are significantly lacking. Although the guidance system described here is primarily intended to facilitate the accurate insertion of biopsy and ablation needles into the liver, similar guidance can be envisioned for staplers or energy devices during transection of liver parenchyma.
In summary, this study demonstrates the feasibility of a magnetic field-based, virtual reality-enhanced guidance system during laparoscopic surgery. The system phenomenally increased the accuracy and precision of complex targeting procedures in our experimental study, for both novices and experts alike. Clinical application of the magnetic field positioning in addition to the already established guidance system would dramatically improve the results of biopsy and ablation procedures and significantly reduce the risk for misdiagnosis and injury. However, the validation of these experiments in an in vivo animal model to gauge their reproducibility in the complex setting of live tissue and under OR conditions is paramount.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Center for Research Resources (NCRR). The prototype guidance system used the Virtual-Reality Peripheral Network (VRPN) library developed by the NIH National Research Resource in Molecular Graphics and Microscopy at the University of North Carolina at Chapel Hill, supported by the NIH NCRR and the NIH National Institute of Biomedical Imaging and Bioengineering. We thank B-K Medical, Inc. and InnerOptic Technology, Inc. for equipment support.
Conflicts of interest
DS, JBM and DAI are unpaid consultants and co-investigators with InnerOptic Technology, Inc. None of the authors have any financial interest in any company or institution that might benefit from this publication.
References
- 1.Siperstein A, Rogers S, Hansen P, Gitomirsky A. Laparoscopic thermal ablation of hepatic neuroendocrine tumour metastases. Surgery. 1997;122:1147–1155. doi: 10.1016/s0039-6060(97)90221-x. [DOI] [PubMed] [Google Scholar]
- 2.Catheline JM, Capelluto E, Turner R, Rizk N, Barrat C, Cazacu F, et al. Comparison of laparoscopic ultrasound and cholangiography during laparoscopic cholecystectomies: results of a prospective study. Gastroenterol Clin Biol. 2000;24:619–625. [PubMed] [Google Scholar]
- 3.Falcone RA, Fegelman EJ, Nussbaum MS, Brown DL, Bebbe TM, Merhar GL, et al. A prospective comparison of laparoscopic ultrasound versus intraoperative cholangiogram during laparoscopic cholecystectomy. Surg Endosc. 1999;13:784–788. doi: 10.1007/s004649901099. [DOI] [PubMed] [Google Scholar]
- 4.Krucker J, Xu S, Glossop N, Viswanathan A, Borgert J, Schulz H, et al. Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol. 2007;18:1141–1150. doi: 10.1016/j.jvir.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation of liver tumours: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla E, Vauthey J-N, Ellis L, Ellis V, Pollack R, Broglio K, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machi J, Sumida K, Limm WM, Hundahl SA, Oishi AJ, Furumoto NL, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumours: percutaneous, laparoscopic and open surgical approaches. J Gastrointest Surg. 2001:477–489. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal M, State A, Lee J, Hirota G, Ackerman J, Keller K, et al. Augmented reality guidance for needle biopsies: an initial, randomized, controlled trial in phantoms. Med Image Anal. 2002;6:313–320. doi: 10.1016/s1361-8415(02)00088-9. [DOI] [PubMed] [Google Scholar]
- 9.State A, Livingston MA, Garrett WF, Hirota G, Whitton MC, Pisano ED, et al. Technologies for augmented-reality systems: realizing ultrasound-guided needle biopsies. 1996. pp. 436–446. ACM SIGGRAPH Conference Series.
- 10.State A, Yang H, Fuchs H, Lee SW, McNeillie P, Burke C. Contextually enhanced 3-D visualization for multi-burn tumour ablation guidance. 2008. pp. 70–77. Proceedings of AMI-ARCS Workshop.
- 11.Beller S, Hunerbein M, Lange T, Eulenstein S, Schlag PM. Image-guided surgery of liver metastases by three-dimensional ultrasound-based optoelectronic navigation. Br J Surg. 2007;94:866–875. doi: 10.1002/bjs.5712. [DOI] [PubMed] [Google Scholar]
- 12.Khadema R, Yeha CC, Sadeeghi-Tehrania M, Baxa MR, Johnson JA, Welch JN, et al. Comparative tracking error analysis of five different optical tracking systems. Comput Aided Surg. 2000;5:98–107. doi: 10.1002/1097-0150(2000)5:2<98::AID-IGS4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Nafis C, Jensen V, Beauregard L, Anderson P. Method for estimating dynamic EM tracking accuracy of surgical navigation tools. Proc SPIE: Med Imaging. 2006;6141:152–167. [Google Scholar]
- 14.Yaniv Z, Wilson E, Lindisch D, Cleary K. Electromagnetic tracking in the clinical environment. Med Phys. 2009;36:876–892. doi: 10.1118/1.3075829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleeman M, Hillebrand P, Birth M, Bruch H. Laparoscopic ultrasound-navigation in liver surgery: technical aspects and accuracy. Surg Endosc. 2006;20:276–279. doi: 10.1007/s00464-005-0196-4. [DOI] [PubMed] [Google Scholar]
- 16.Krucker J, Viswanathan A, Borgert J, Glossop N, Yang Y, Wood B. An electromagnetically tracked laparoscopic ultrasound for multi-modality minimally invasive surgery. Int Congr Ser Comput Assist Radiol Surg. 2005;1281:746–751. [Google Scholar]
- 17.Dagher I, O'Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856–860. doi: 10.1097/SLA.0b013e3181bcaf46. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection – 2804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen KT, Geller DA. Is laparoscopic liver resection safe and comparable to open liver resection for hepatocellular carcinoma? Ann Surg Oncol. 2009;16:1765–1767. doi: 10.1245/s10434-009-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 21.Cash DM, Miga MI, Glasgow SC, Dawant BM, Clements LW, Cao Z, et al. Concepts and preliminary data toward the realization of image-guided liver surgery. J Gastrointest Surg. 2007;11:844–859. doi: 10.1007/s11605-007-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herline AJ, Stefansic JD, Debelak JP, Hartmann SL, Pinson CW, Galloway RL, et al. Image-guided surgery: preliminary feasibility studies of frameless stereotactic liver surgery. Arch Surg. 1999;134:644–649. doi: 10.1001/archsurg.134.6.644. discussion 649–650. [DOI] [PubMed] [Google Scholar]
- 23.Oldhafer KJ, Stavrou GA, Prause G, Peitgen HO, Lueth TC, Weber S. How to operate a liver tumour you cannot see. Langenbecks Arch Surg. 2009;394:489–494. doi: 10.1007/s00423-009-0469-9. [DOI] [PubMed] [Google Scholar]
- 24.Catheline JM, Turner R, Rizk N, Barrat C, Champault G. The use of diagnostic laparoscopy supported by laparoscopic ultrasonography in the assessment of pancreatic cancer. Surg Endosc. 1999;13:239–245. doi: 10.1007/s004649900954. [DOI] [PubMed] [Google Scholar]
- 25.Goletti O, Celona G, Galatioto C, Viaggi B, Lippolis PV, Pieri L, et al. Is laparoscopic sonography a reliable and sensitive procedure for staging colorectal cancer? A comparative study. Surg Endosc. 1998;12:1236–1241. doi: 10.1007/s004649900827. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi M, Torzilli G, Machi J. History of intraoperative ultrasound. Ultrasound Med Biol. 1998;24:1229–1242. doi: 10.1016/s0301-5629(98)00112-4. [DOI] [PubMed] [Google Scholar]
- 27.Hummel JB, Bax MR, Figi ML, Kang Y, Maurer C, Jr, Birkfellner WW, et al. Design and application of an assessment protocol for electromagnetic tracking systems. Med Phys. 2005;32:2371–2379. doi: 10.1118/1.1944327. [DOI] [PubMed] [Google Scholar]


