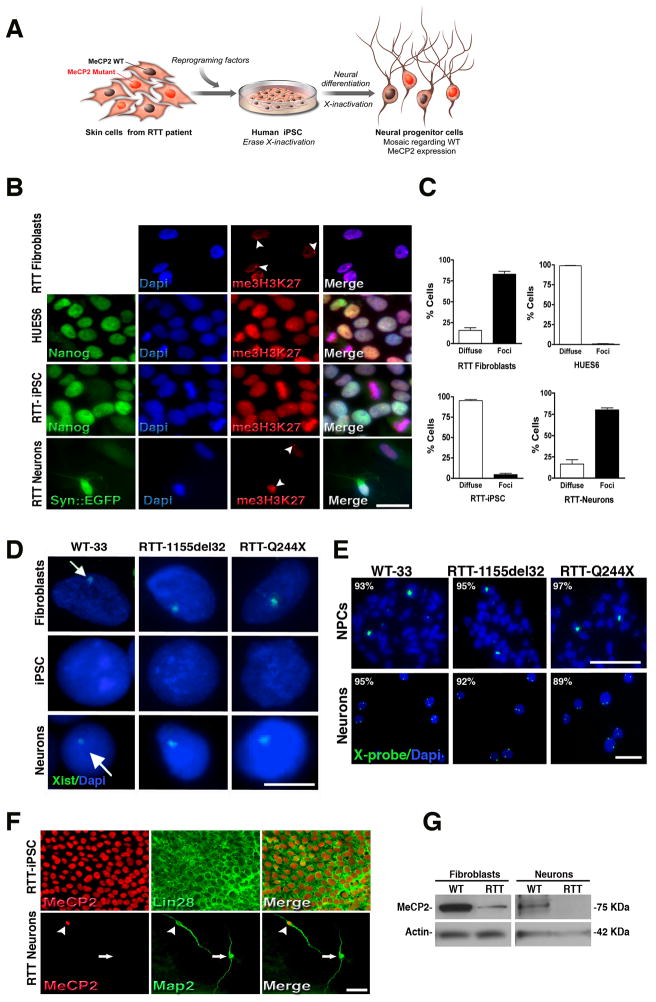
Figure 3.
RTT-iPSC clones undergo X-inactivation during differentiation. (A), Schematic representation of X-inactivation dynamics during reprogramming and further neural differentiation. RTT fibroblasts are mosaic for the MeCP2 WT gene expression. During reprogramming, X-inactivation is erased and iPSCs express both MeCP2 alleles. Upon neuronal differentiation, X-inactivation is re-established and the resultant cells are mosaic for MeCP2 WT gene expression. (B), Immunofluorescence for me3H3K27 in fibroblasts, pluripotent cells (Nanog-positive) and after neuronal differentiation (Syn::EGFP-positive). Pluripotent cells (hESCs and iPSCs) show diffuse staining whereas differentiated cells (fibroblasts and neurons) exhibit prominent me3H3K27 nuclear foci (arrowheads). Cells were counterstained with Dapi. Bar = 15 μm. (C), Quantification of cells with diffused or foci me3H3K27 nuclear staining. Data shown as mean ± s.e.m. (D), RNA FISH shows that Xist RNA domains are present in the original fibroblasts before reprogramming. iPSCs show no Xist expression. Neurons derived from normal and RTT iPSCs show clear Xist clouds, indicating transcriptional silencing of the X chromosome (arrows). Bar = 5 μm. (E), Two DNA FISH signals are evident in the nuclei of iPSC-derived NPCs and neurons, revealing the presence of two X chromosomes. Bar = 10 μm. (F), RTT-iPSCs (1155del32) expressed WT MeCP2 but derived neurons displayed mosaicism regarding WT (arrowhead) and mutant (arrow) MeCP2 forms. Bar = 50 μm. (G), RTT-derived fibroblasts and neurons have reduced levels of WT MeCP2 protein by Western blot. See also Figure S3.