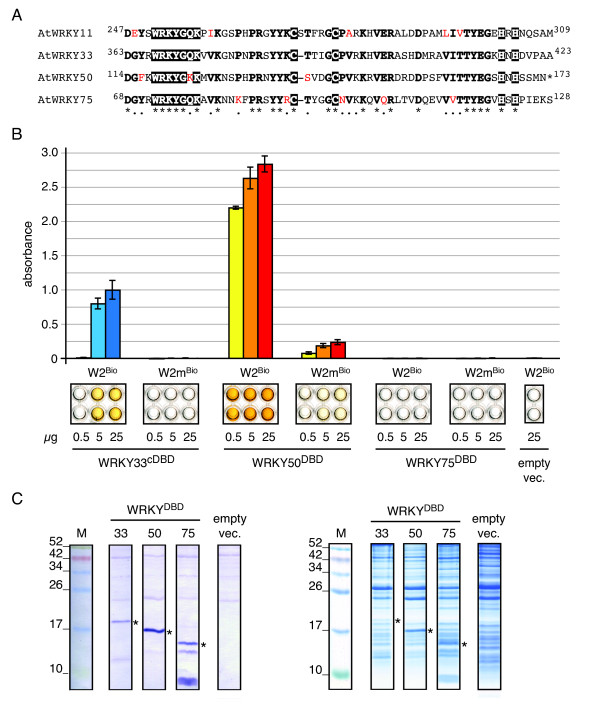
Figure 3.
DNA-binding capacity of Arabidopsis thaliana WRKY33cDBD, WRKY50DBD and WRKY75DBD to the W2-probe. A. Amino acid alignment of WRKY11, WRKY33, WRKY50 and WRKY75 DNA-binding domain (DBD) sequences. The highly conserved WRKY-consensus and the zinc-finger are highlighted (white on black); conserved amino acid residues are displayed in bold face. Non-conserved residues that might contribute to differences in WRKY-domain function by altering the binding specificities are highlighted in red. B. DPI-ELISA results for AtWRKY33cDBD, AtWRKY50DBD and AtWRKY75DBD binding to the W2- or W2m-probes. Different amounts of extracts (0.5, 5, 25 μg total protein per well) were examined with W2Bio- and W2mBio-probes. Representative wells of the microtiter plate are shown below the graph for visual inspection. C. Detection of the immobilized His-epitope tagged proteins with anti-His-antibodies in the crude extract by western blotting using (left). Asterisks indicate the appropriate bands (AtWRKY33cDBD - 20 kDa, AtWRKY50DBD - 17 kDa, AtWRKY75DBD - 15 kDa). Coomassie-stained SDS-PAGE (right) is shown for equal loading of the gel.