Summary
HDAC inhibitors (HDACI) are now emerging as one of the most promising new classes of drugs for the treatment of select forms of non-Hodgkin’s lymphoma (NHL). They are particularly active in T-cell lymphomas, possibly hodgkin’s lymphoma and indolent B cell lymphomas. Presently, two of these agents, vorinostat and romidepsin, have been approved in the US for the treatment of relapsed and refractory cutaneous T cell lymphomas (CTCL). Initially, these agents were developed with the idea that they affected transcriptional activation and thus gene expression, by modulating chromatin condensation and decondensation. It is now clear that their effects go beyond chromatin and by affecting the acetylation status of histones and other intra-cellular proteins, they modify gene expression and cellular function via multiple pathways. Gene expression profiles and functional genetic analysis has led to further understanding of the various molecular pathways that are affected by these agents including cell cycle regulation, pathways of cellular proliferation, apoptosis and angiogenesis all important in lymphomagenesis. There is also increasing data to support the effects of these agents on T cell receptor and immune function which may explain the high level of activity of these agents in T cell lymphomas and hodgkin’s lymphoma. There is ample evidence of epigenetic dysregulation in lymphomas which may underlie the mechanisms of action of these agents but how these agents work is still not clear. Current HDAC inhibitors can be divided into at least four classes based on their chemical structure. At present several of these HDAC inhibitors are in clinical trials both as single agents and in combination with chemotherapy or other biological agents. They are easy to administer and are generally well tolerated with minimal side effects. Different dosing levels and schedules and the use of isospecific HDAC inhibitors are some of the strategies that are being employed to increase the therapeutic effect of these agents in the treatment of lymphomas. There may also be class differences that translate into specific activity against different lymphoma. HDAC inhibitors will likely be incorporated into combinations of targeted therapies both in the upfront and relapsed setting for lymphomas.
Keywords: Histone deacetylase inhibitors, Non-Hodgkin lymphoma, T-cell lymphoma, Epigenetics
Introduction
Epigenetic modifications are encompassed by one of three distinct biological processes: (1) acetylation and deacetylation of histones catalyzed by histone acetyltransferases (HAT) and histone deacetylases (HDAC); (2) genome methylation; and (3) small silencing RNA (siRNA). The first two of these pathways have emerged in recent years as a promising new strategy for the treatment of many different hematologic malignancies, with HDAC inhibitors (HDACI) now emerging as one of the most promising new classes of drugs for the treatment of select forms of non-Hodgkin’s lymphoma (NHL). Presently, two HDACI, vorinostat and romidepsin, have been approved in the US for the treatment of relapsed and refractory cutaneous T cell lymphomas (CTCL) [1, 2]. By affecting the acetylation status of histones and other intra-cellular proteins, drugs inhibiting HDAC can modify gene expression and cellular function [3]. Initially, these agents were developed with the idea that they modulated transcriptional activation and thus gene expression, by modulating chromatin condensation and decondensation. More recently, it has been recognized and now generally accepted, that the effects of HDACI go well beyond chromatin, and includes a plethora of effects on the post-translational modification of many intracellular proteins. Though the fundamental mechanisms of HDACI inhibitor action are discussed in other chapters, it is worth highlighting particular concepts that will be germane to understanding these agents in B- and T-cell lymphoma.
DNA is packaged around a core of eight histone proteins (a pair of histones 2A, 2B, 3 and 4) into discrete units called nucleosomes [4]. Acetylation of the ε-amino moieties on the lysine tails of the histone leads to an open, or transcriptionally active decondensed state of chromatin which is accessible to transcription factors, and thus allows for transcription of various genes. The condensed or closed chromatin state, catalyzed by the deaceytlation of the lysine tails, prevents access of the transcription factors to the structure of the DNA leading to transcriptional silencing. These reactions are catalyzed by two major classes of enzymes referred to as HATs and HDAC. HDAC inhibitors block these HDAC enzymes, and thus maintain the chromatin in an acetylated and transcripttionally active state. Histones themselves also undergo many different types of post-translational modifications though most is probably known about acetylation as described above. Emerging insights into other post-translational modifications like methylation suggest these reactions may play equally important roles in governing transcription, and may be an equally valid target for drug development.
To date over 18 different HDACs have been identified, being grouped into four classes based on their homology to yeast proteins, as shown in Table 1. In brief, these include: (1) Class I enzymes consisting of HDAC1, HDAC2, HDAC3, HDAC8, (2) class II enzymes consisting of HDAC4, HDAC5, HDAC7, HDAC9, HDAC6, HDAC10; and (3) class IV enzymes consisting of HDAC11. These HDACs all require a Zn molecule in their active site and are inhibited by what are often referred to as ‘pan’ HDAC inhibitors [5]. The unique class III HDAC, also known as sirutins (Sirutin 1-7), are homologous to yeast Sir 2 protein, require NAD+ as a coenzyme for their activity and are not affected by the pan HDAC inhibitors [6].
Table 1.
Classification of histone deacetyalse inhibitors
| Class | Targeted enzymes | Required co factor |
|---|---|---|
| I | HDAC 1,2,3,8 | Zn2+ |
| II | HDAC 4,5,7,9, 6, 10 | Zn2+ |
| III | SIRTUINS 1-7 | NAD+ |
| IV | HDAC 11 | Zn2+ |
The biological importance of histone deacetylases has been demonstrated in mouse knockout models. Genetic deletions of class I enzymes [7] are lethal in the embryonic and perinatal stages of development, whereas class II [8] knockouts produce viable progeny, though they do exhibit significant developmental abnormalities. The pharmacologic inhibition of HDAC leads to several downstream effects in tumor cell lines which can be categorized as follows [9]: (1) cell cycle arrest in G1-S phase; (2) induction of apoptosis mediated by effects on pro- and antiapoptotic mechanisms of cell death; (3) inhibition of angiogenesis; (4) effects on the endoplasmic stress response; (5) effects on the activation or inactivation of tumor suppressor genes or oncogenes involved in growth control and cell death. This confirms that the pharmacologic inhibition of histone deacetylases can lead to pleiotropic effects on signaling pathways that affect proliferation, differentiation, angiogenesis and cell survival [10–12]. Uniquely, the Class III HDAC enzymes (also referred to as the sirutins), together with NAD+, lead to the production of the deacetylated peptides, nicotinamide, and ADP ribose. Nicotinamide is a negative regulator of this reaction and thus an inhibitor of class III HDACs. Sirutins couple the processes of lysine acetylation and deacetylation to the metabolic NAD/NADH+ pathway and have been shown to be important in the regulation of transcription, recombination, cell division, cell cycling, microtubule assembly, and cellular responses to stress and DNA damaging agents [6].
Dysregulation of Epigenetic pathways in lymphoproliferative malignancies
While there is still much to learn regarding the basic biological implications of modifying HATs and HDAC enzymes, it is clear that they control many essential aspects of normal and abnormal cell behavior. Inhibition of HDAC is associated with a pleiotropic set of consequences, many of which are likely to vary as a function of the HDAC inhibitor, the cellular context, and perhaps even the concentration or dose of a specific agent. New concepts that are emerging regarding the importance of this biology on carcinogenesis have focused on the balance of HAT and HDAC activity [13]. For example, the tight control of the acetylation or deacetylation of key proteins, similar to our understanding of phosphorylation and control of the kinome, can have profound effects on the activation or inactivation of important transcription factors like E2F, p53, NF-KB, HIF alpha, estrogen receptor, signaling pathway proteins like STAT3, chaperone proteins like hsp-90, DNA repair enzyme like Ku70 and structural proteins like alpha tubulin. The wide range of proteins affected by the many different HDAC enzymes, coupled with the many new drugs now targeting this biology, suggests we may only be at the beginning in figuring out how to manipulate the ‘acetylome’ in a fashion that will both prevent and treat diverse forms of cancer through epigenetic modulation [14].
The promising activity of HDAC inhibitors in certain types of lymphomas has prompted investigators to look at the expression of HATs, HDACs and other epigenetic markers in different types of lymphomas to pinpoint a mechanisms of action and a rationale for the observed anti lymphoma effect. The results have only shown that the clinical effect is at best empirical at the moment. Some of this data is discussed below.
CTCL has been the disease with the highest clinical response rates with HDAC inhibitors prompting studies of HDAC expression and other epigenetic markers in CTCL to look into the molecular basis of this responsiveness. Marquard et al. [15] have evaluated the expression of HDAC1, HDAC2, HDAC6 and acetylated H4 by immunohistochemistry in 73 samples of CTCL obtained from patients with variable clinical courses ranging from indolent to aggressive and correlated their findings with survival in this cohort of patients. Their results have shown clear differences in the expression of certain markers within certain subgroups of the disease but have not provided any insight into the therapeutic benefit seen with epigenetic therapy. When comparing aggressive versus indolent cases, no difference was found in the expression of HDAC1, and HDAC6 but the expression of HDAC2 and acetylated H4 was found to be higher in aggressive disease compared to indolent (HDAC2—55.5%—aggressive CTCL vs 15% indolent, acetylated H4—22% aggressive vs 8% indolent). Survival correlated with the over expression of HDAC-6 (p = 0.04, hazard ratio 0.39) independent of CTCL subtype. Dysregulation of other epigenetic pathways particularly promoter site hypermethylation has been identified in many lymphoid malignancies including CTCL. Van Doorn et al. [16, 17] compared genome-wide DNA methylation screening of CTCL samples with benign skin disorders and carried out methylation profiling using DNA isolated from patient specimens including those with transformed disease. In the initial screens, widespread promoter hypermethylation suggestive of epigenetic instability was demonstrated in malignant T-cells in CTCL. At least 35 CpG islands were hypermethylated in tumor stage CTCL samples including that of the putative tumor suppressor gene BCL7a (B-cell CLL/Lymphoma 7) in 48% of samples, PTPRG gene (protein tyrosine phosphatase receptor gamma) in 27% of samples and THBS4 gene (thrombospondin 4) in 52% of the samples. These genes were also hypermethylated in the CTCL cell line Myla but not in the control samples. Treatment of this cell line with a hypomethylating agent 5-aza-2- azacitidine resulted in re-expression of these genes as detected by quantitative PCR. Bcl-7 is of particular interest because a higher frequency of hypermethylation of BCL-7 (and inactivation) was observed in samples from patients with more aggressive disease (64%) compared to indolent CTCL (14%). BCL-7, which is located on chromosome 12q 24.31 has been cloned as part of the chromosomal translocations seen in Burkitt’s lymphoma and is at a site of frequent breakpoints in lymphoma [18]. While the exact cellular function of BCL7a is unknown at present, its expression has been shown to be diminished in mycosis fungoides and PTCL compared with lymphoblastic lymphoma indicating possible contribution to lymphoid malignancies arising from mature T cells. PTGR is a member of the tyrosine phosphatase gene superfamily, and is commonly silenced across many types of human malignancies [19], while THBS4 encodes for an extracellular calcium binding protein involved in proliferation, adhesion and migration and has been reported to be hypermethylated in other cancers besides CTCL [20]. Other well known tumor suppressor genes shown to have promotor hypermethylation in 21 CTCL samples as compared to samples from normal skin in volunteers included p73 (48%), p16 (33%), CHFR (19%), p15 (10%) and TMS1 (10%). The CpG islands of the DNA repair gene MGMT gene were hypermethylated in all tested CTCL samples as a well as the three control samples. In general, these hypermethylated genes can be grouped into the following functional categories: cell cycle dysregulation (p15, p16, p73), defective DNA repair (MGMT), apoptosis dysregulation (TMS1, p73) and chromosomal instability (CHFR). p73 is responsible for activation induced death of T-cells and its silencing leads to accumulation of T cells in tissues, an important step in the pathogenesis of CTCL [21–24]. Hypermethylation of p73 has also been described in nodal B-cell and NK-cell lymphomas [24]. Promoter hypermethylation of p16 has been demonstrated in many lymphoid malignancies, including CD30+ T-cell NHL. CHFR encodes for a protein regulating the mitotic checkpoint pathway that regulates the transition to metaphase and its silencing may contribute to chromosomal instability [25–27]. Hypermethylation of p16 and MLH1 gene have also been detected in patch/plaque MF [26–28] indicating their contribution to pathogenesis in early stages of CTCL.
Epigenetic dysregulation has also been studied in other types of lymphoma and there is evidence that HDAC expression can be variable depending on the type and aggressiveness of the disease. Using western blots and immunohistochemistry, Gloghini et al. [29] have studied the expression of several class I and II HDACS in a diverse panel of lymphoma cell lines, primary samples of benign reactive lymph nodes (n = 5), as well as biopsy samples of various lymphomas (DLBCL n = 171, T cell n = 5, Hodgkin lymphoma n = 22, and other B cell lymphomas n = 152). All 14 lymphoma cell lines uniformly expressed the class 1 HDAC enzymes (1,2,3,8) but there was significant variability in the expression of class II enzymes. In particular HDAC 6 expression was high in mantle cell lymphoma, DLBCL and T-cell derived lymphoma cell lines but low in multiple myeloma, Hodgkin lymphoma and anaplastic large cell lymphoma cell lines. The class IV HDAC 11 was expressed in all cell lines. Subsequently, the authors compared the expression of HDACs in normal reactive nodes to the pattern of expression seen in primary lymphoma samples to look for any differences that may contribute to lymphomagenesis. Within reactive lymph nodes there was almost uniform expression of class I HDACs in all cellular compartments. But consistent with the cell line data, HDAC2 enzymes were differentially expressed in cellular compartments with HDAC10 present in all cellular compartments and HDAC6 expression restricted to plasma cells. The relative expression of HDACs has been studied across many subtypes of lymphoma within primary samples from lymphoma patients. Class I HDACs 1,2,3, 8 were expressed in all NHL and HL cases. Class II HDAC expression was more variable, with class 10 being present in all cases of NHL and classical HL, while HDAC6 being present in B cell lymphomas with plasmacytoid/ plasmablastic differentiation. Interestingly, only two of 52 cases of DLBCL expressed HDAC6 which was absent in all 22 cases of HL. The class IV HDAC 11 was expressed in all lymphoma cell lines, all samples of NHL and in none of the four HL cases that were tested. The importance of this variability becomes apparent in the following set of observations. Villagra et al. [30] have shown that over expression of HDAC11 inhibits interleukin 10 expression and induces inflammatory antigen presenting cells that are able to prime naïve T-cells and restore the responsiveness of tolerant CD4+ cells. Disruption of HDAC11 in APCs leads to the upregulation of expression of the gene encoding for IL-10 leading to impairment of T-cell responses. Hence, aberrant expression of HDACs in the microenvoirnment of lymphoma may also be important for immunomodulation and antitumor immune responses [31] and may lead to opportunities for therapeutic interventions.
This data clearly points to the variability in the expression of HDACs within different tissue and tumor types even though these differences may not be fully defined yet. These observations remain important as we refine our efforts to design even more selective HDAC inhibitors, and sort out the best disease contexts within which to explore their activity. Similar to the proteasome inhibitors, it remains unlikely that developing the best isoselective HDAC inhibitor will be the optimal strategy to move these agents forward, but rather understanding the down-stream consequences of inhibiting specific HDAC enzymes coupled with our understanding of how addicted any given malignancy is to that particular biology, may prove the optimal rationale for matching the drug to the disease. It is also likely that pan-HDAC inhibition may afford coverage of a broader spectrum of biology that may be associated with even greater benefit in comparison to more isoselective inhibitors.
Recently, the Class II HDAC6 has gained a lot of attention. As mentioned above, HDAC6 is one of the most variable HDAC enzymes. It is known to affect the acetylation status of several proteins including alpha—tubulin, and is important in the regulation of microtubule stability and function. In addition, it may serve as a molecular chaperone, and plays a role in regulating the aggresome, a proteosome independent pathway that eliminates misfolded protein [32] shown to be important in some cancers. Interestingly, mice knocked out for HDAC6 show hyperacetylated tubulin in most tissues but remain viable and fertile with normal lymphoid development [33] This linkage between inhibition of an HDAC and the proteasome pathway has now raised a strong mechanistic rational for the combination of these agents in the clinical setting, much of which is centered around the unfolded or misfolded protein response. Misfolded proteins are thought to be degraded either via the ubiquitin-proteosome pathway, and possibly through the aggresome. Misfolded proteins destined for the aggresome are thought to be transported along microtubules via the activity of motor proteins like dyne and adapters proteins like HDAC6, to the microtubule organizing center (MTOC), which in turn transports these proteins to the lysosome for degradation as part of the HDAC-6—aggresome pathway. Pharmacologic inhibition of HDAC-6 results in hyperacetylation of tubulin and disruption of the aggresome mediated pathway resulting in apoptosis, which may explain the mechanistic basis for their synergy with proteosome inhibitors [29, 34]. It should be pointed out, that there is no evidence that inhibiting one HDAC enzyme over another is associated with improved activity, nor is there evidence that more selective HDAC inhibitors will be associated with an improved adverse effects profile.
Molecular pharmacology of histone deacetylase inhibitors
Inhibition of HDACs is known to be associated with myriad effects on tumor cells, which has made it exceedingly difficult to establish a true mechanistic pathway of how these agents work in lymphoma, and T-cell lymphomas in particular. Biochemically, HDAC inhibitors maintain histones and other intracellular proteins in an acetylated state. These biochemical modifications are known to be associated with a variety of downstream biological effects including the activation or inactivation of transcription, influences on cell cycle regulation and apoptosis, and many pro-differentiating effects. Gene expression profiling (GEP) of cells that have been treated with HDAC inhibitors has shown that up to 22% of the genes in the genome can be modulated with these agents within as early as 4 h post-treatment [35, 36]. Efforts to identify a discrete mechanistic role for any specific HDAC inhibitor based on the alteration of a specific gene expression profile remains elusive. The major effects of HDAC inhibition on cancer cells are grouped as follows:
Effects of HDAC inhibitors on cell cycle control and proliferation
Among the many pleiotropic effects that HDAC inhibitors have on cells, perhaps the most is known about their effects on cell cycle regulation and control. It is well established that HDAC inhibitors block cell proliferation and cause apoptosis in cell lines derived from both hematologic and solid tumor malignancies by causing cell cycle arrest in G1 or G2/M mainly by altering the expression of proteins (cyclins, cyclin dependent kinase inhibitors,) involved in growth control [37]. HDAC inhibitors repress the expression of cyclin D [38, 39] and cyclin A which reduces the activity of CDK4 and CDK2 leading to cell cycle arrest in G1. HDAC inhibitors also cause up-regulation of p21, p27 and p16 leading to inhibition of cell cycle progression after binding to and inactivating CDK4 and CDK2 [40, 41]. The consistently observed increase in p21 expression is considered to be one of the most important mechanisms leading to HDAC inhibitor mediated G1/S arrest [42]. As clinical efficacy of HDAC inhibitors gets established in the treatment of lymphomas, the field is now poised to study specific HDAC inhibitors for their effects in specific subtypes of lymphoma in order to identify discrete differences seen in the epigenitc modifications produced by the different agents. Vorinostat has been shown to cause acetylation of histones H2B, H3, and H4 associated with increased expression of p21 resulting in G2 arrest and apoptosis in CTCL and HL cell lines [43]. Peart et al. [35] have used GEP and small interfering RNA (siRNA) experiments to look at the effect of two recently approved HDAC inhibitors, vorinostat and romidepsin on gene expression in T-cell lymphoblastic cell lines (CEM, Jurkat). Cell lines exposed to either of these agents resulted in changes noted in a highly overlapping set of up to 22% of the genes in the cells within a 16 h culture period. Cyclin D was consistently down regulated by vorinostat, whereas the expression of several other cyclins, including G1, G2, E1, E2, F and B2, as well as their inhibitors CDK2m CDK2N2, and CDK2AP1), were affected by both HDAC inhibitors. Ellis et al. [44] studies the patterns of gene expression in patients with CTCL pre- and post-treatment with panobinostat. They demonstrated a consistent downregulation of Cyclin D1 which was confirmed by RT-PCR. Other mechanisms of action that lead to cell cycle arrest have been demonstrated in cell lines where vorinostat, trichostatin A, and MS-27-275 have all been shown to result in transcriptional downregulation of enzymes important in nucleotide synthesis. These effects were seen primarily in CTP synthetase and thymidylate synthetase, and were associated with tumor cell death by inducing cell cycle arrest in S phase [45]. Butyrate and TSA, two aliphatic acid HDAC inhibitors, have been shown to induce GADD45 alpha and beta subunits (a stress induced gene downstream of p53) in colon cancer cell lines which may contribute to the G2/M arrest [46].
There is increasing evidence that drugs targeting HDAC can affect several pathways that are important in cell proliferation and differentiation of lymphoid cells, including the b-TGF pathway, the JAK-STAT pathway, myc and Bcl-6 [35]. Peart et al. [35] have demonstrated that following exposure to HDAC inhibitors, there was a rapid increase in the expression of a number of antiproliferative genes including TOB1, TOB2, BTG2, that all belong to a family of genes important in neuronal differentiation. Members of this family are p53 inducible, are activated in conditions of genotypic stress and can impair G1-S progression. The oncogene c-myc, which is known to be dysregulated in several lymphomas, in particular in Burkett’s lymphoma through the t (8–14), was shown to be downregulated after exposure of the malignant cells to vorinostat and romidepsin [35]. At the same time, three members of the same myc family (i.e. MXII, MAD, and MLX) were up regulated. Typically, MAD competes with MAX and inhibits the transcriptional activity of MYC leading to the hypothesis that treatment with HDAC inhibitors can produce a loss of MYC driven transactivation and cell proliferation. Another important gene that is upregulated by both vorinostat and romidepsin is the TIEG (TGF Beta inducible early response genes), which is upstream of the type B-TGF pathway and is involved in mediating responses that control cellular differentiation and apoptosis via the TGF-B pathway [47]. Similarly the ID ((inhibitor of differentiation) genes, ID2 and ID3 that induce lymphocyte growth arrest and apoptosis are also rapidly upregulated by HDAC inhibitors [48] and may result in cell death. Bcl-6 is a gene that encodes for a protein that acts as a sequence-specific repressor of transcription, and has been shown to modulate the transcription of STAT-dependent Interleukin 4 (IL-4) responses of B cells. BCL-6 is frequently translocated and hypermutated in diffuse large B cell lymphoma (DLCL), and serves as a transcriptional repressor inhibiting p53 and other genes involved in the control of lymphocyte activation, differentiation and apoptosis. Acetylation of Bcl-6 by HDAC inhibitors abrogates this effect allowing the genes repressed by Bcl-6 to be activated [49, 50]. Panobinostat, another hydroxamic acid HDAC inhibitor, has been shown to suppress proliferation genes including NR2F2, Cyclin D and TM4SF18 [44] as demonstrated by gene expression in tissue obtained from skin samples of the patients CTCL both before and after treatment. The direct effect of HDAC inhibitors on non-histone proteins can also affect the growth and differentiation of cells. These proteins are broadly grouped as transcriptional coregulators (e.g. RB, MSL-3, DEK, PGC-1α), DNA binding transcriptional factors (e.g. BCL-6, p53, c-Myc, E2F, GATA, Ying Yang, NF-κB, CREB, IRF), signaling mediators (e.g. STAT-3, IRS-1), chaperone proteins (e.g. HSP90), steroid receptors (e.g. androgen, estrogen, and glucocorticoid), and DNA repair enzymes (e.g. WRN, KU70, and FEN1) [35, 51–57]. The STAT family of transcription factors is particularly important because of their important role in lymphomagenesis. In particular STAT 3 is found to be constitutively activated in CTCL, HL and several other lymphomas [58]. After treatment with vorinostat, p-STAT-3, localized to the cytoplasm from the nucleus in patient samples with CTCL who had responded [59]. Since nuclear translocation is essential to the function of transcription factors, this shift maybe indicative of inactivation of STAT 3 thus correlating with the antilymphoma effect of vorinostat.
Effects of HDACI on survival pathways
Many lines of preclinical data have established a direct correlation between apoptotic tumor cell death and therapeutic efficacy of HDAC inhibitors. Gene expression profiling has revealed that treatment of cells with HDAC inhibitors results in expression of proteins that favor a pro-apoptotic response and suppresses anti-apoptotic mechanisms [60, 61]. HDAC inhibitors-induce death of tumor cells through the induction of both the internal and external pathways of apoptosis, as well as through non-apoptotic mechanisms like autophagy [62]. The specific evidence regarding details of these effects are described as follows:
Extrinsic apoptotic pathway
The extrinsic apoptotic pathway designed to induce apoptosis in response to extra cellular stimuli is induced by engagement of the DISC (death induced signaling complex) via cell surface receptors and their interaction with ligands that ultimately lead to caspase 8 activation [63, 64] and apoptotic death via the final common pathway. Like all other activities in the cell, this is controlled by several sets of opposing protein pathways that determine the fate of the cell and are modulated by HDAC inhibitors. Specifically, HDAC inhibitors have been shown to increase the expression of genes that encode for death receptors and their ligands such as Fas and the Apo 2L/TRAIL receptors, DR4 and DR5, and to downregulate c-FLIP, c1AP2, and X1AP that inhibit the death receptor pathway [65]. The use of neutralizing antibodies and siRNAs that target TRAIL, Fas, or over expression of cFLIP can protect tumors form HDAC inhibitor induced apoptosis, confirming the mechanistic role of the extrinsic apoptotic pathway in HDAC inhibitor induced cell death.
Intrinsic apoptotic pathway
The intrinsic apoptotic pathway gets activated from internal cellular stimuli that result in increased mitochondrial permeability and release of cytochrome c, Smac, and Omi into the cytosol via the mitochondrial voltage-dependent anion channel (VDAC) and are coupled with the induction of Apaf-1 (apoptosis pathway activating factor that leads to caspase 9 and 3 activation and activation of the final common pathway of apoptosis. The tendency towards intrinsic apoptosis is tightly managed by a litany of pro- and anti-apoptotic proteins ( Bcl-2, Bcl-xL, NOXA), as well as the BH3 only proteins like Bid, Bik and Bim. Treatment of cells with HDAC inhibitors results in perturbation of this balance shifting the cell towards or away from the induction of apoptosis [66, 67]. Interestingly, the over expression of Bcl2 and siRNA-mediated knockdown experiments of Bad, Bim, and Noxa reduces the apoptotic effects of HDAC inhibitors. It is now becoming increasingly important that any potential differences in the biological activity between specific HDAC inhibitors, or even classes of HDAC inhibitors (say for example aliphatic acids vs hydroxamic acids vs natural product macrolides like romidepsin) be established. Vorinostat and romidepsin are two HDAC inhibitors that have been approved for clinical uses and are the most extensively studied in this regard. They differ in their chemical structure as well as their potency regarding in-vitro cytotoxicity assays and their respective EC50 for the different HDAC enzymes. Both agents have been shown to induce caspase dependent apoptosis in lymphoma cell lines derived from Eu-myc mouse models and xenograft mouse models of mice bearing Eu-myc lymphoma tumors show increased survival and absence of tumor at autopsy following treatment with vorinostat or romidepsin [35]. However, these agents differ with respect to their effects on specific apoptotic and antiapoptotic proteins that may lead them to have differing therapeutic implications. Knockout mice models of specific proteins in the apoptotic pathways have been used to delineate the specific mechanisms of apoptosis for each of these agents [68, 69]. Vorinostat induces apoptosis in a p53 independent manner but was unable to kill cells that over expressed Bcl-2 (as is seen in many human B cell lymphomas) and Bcl-XL in this model system. Interestingly, over expression of Bcl-2 conferred resistance to apoptosis mediated by other HDAC inhibitors as well, including LAQ824, LBH589, valproic acid and MS-275 where as romidepsin induced apoptosis in cells that over expressed Bcl-2 but not those over-expressing Bcl-xL, suggesting that a unique difference between these agents. Both Bcl-2 and Bcl-xL are anitapoptotic proteins that function by interacting with Bax and Bak, and thus work collaboratively to prevent mitochondrial membrane depolarization and induction of apoptosis. Bcl-xL may be may be more critical in different stages of B-cell ontogeny (i.e. more important in immature B cells for example) and ten times more effective than Bcl-2 in inhibiting apoptosis mediated by chemotherapeutic agents like doxorubicin and etoposide, whereas Bcl-2 may be more important in more mature B-cells (i.e. follicular lymphoma for example) [70, 71]. This and other reports have highlighted other potentially important differences between vorinostat and romidepsin, including: (a) the cleavage and activation of the BH3—only protein Bid occurs in a caspase independent manner following treatment with vorinostat but is dependent on caspase activition in response to romidepsin; (b) the multidrug resistant gene P-glycoprotein can inhibit apoptosis induced by romidepsin whereas it has no effect on vorinostat. Another hydroxamic acid panobinostat has been shown to affect genes involved in apoptosis including Septin 10, TEF and SORBS2 [44].
In addition to these modulatory effects on the proteins governing apoptosis, the antiapoptotic effects of HDAC inhibitor can also be mediated by several indirect mechanisms that lead to cell death by apoptosis. One such important pathway involves the generation of reactive oxygen species (ROS) [72] that lead to increased mitochondrial permeability and consequent activation of the apoptotic pathway. HDAC inhibitors have been shown to induce ROS and treatment of cells with ROS scavenging molecules like N-acetyl cysteine and thioredoxin can lead to protection of the cell against HDACI mediated apoptosis. Post-translational modification of proteins through the maintenance of an acetylated state of proteins like Bid, thioredoxin, and regulators of ROS may be an additional mechanism contributing to apoptosis [73].
Autophagy
HDAC inhibitors also induce non-apoptotic cell death possibly by autophagy. Blockade of caspase-mediated apoptosis bya pan-apoptosis inhibitors like z-vad still induces to cell death, suggesting that other non-apoptotic can be invoked following HDAC inhibition, which most reports now believe is likely to be mediated by autophagy, although the precise mechanism is not yet known [74].
By understanding the specific effects of the different HDAC inhibitors on apoptosis and cell cycle control for example, it will be possible to conceptualize rational drug: drug combinations that will take advantage of the underlying molecular pharmacology to develop likely synergistic platforms in a very cell context focused manner.
Antiangiogenic effects
HDAC inhibitors have been shown to have anti-angiogenic effects that appear to be mediated by various mechanisms including upregulation of angiogenesis inhibitors like thrombospondin, Von-Hippel Landau factor, as well as down regulation of factors that promote vasculogenesis including vascular endothelial growth factor (VEGF) and HIF-a (hypoxia induced protein). HDAC inhibitors also downregulate the expression of survivin, an anti-apoptotic protein in endothelial cells, thereby inhibiting the growth of vascular endothelium [75, 76] and interfering with vasculogenesis. Tumor samples from patients treated with vorinostat have shown clear evidence of decreased vascularity consistent with this observation [59]. A number of genes that are known to affect angiogenesis including the proangiogenic ANGPT1 and guynalate cyclase 1A3 (GUCY1A3) and were also found to be downregulated after treatment with panobinostat [44].
Clinical pharmacology of histone deacetylase inhibitors
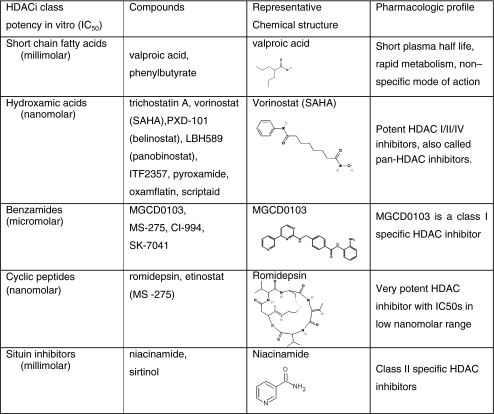
HDACI are a chemically diverse group of naturally occurring and synthetic molecules found to inhibit the activity of HDACs in a wide range of concentrations from low nM to high mM [13, 77] as listed in Table 2. Presently, there are at least 18 different HDAC inhibitors being evaluated in preclinical and early clinical trials that target the Zn dependent (i.e. Class 1 and 2) HDACs, including two agents already approved for the treatment of CTCL in the U.S., namely vorinostat and romidepsin. Table 3 lists all current HDAC inhibitors undergoing clinical evaluations. Most HDAC inhibitors have a short plasma half life (vorinostat- 2 h [78], romidepsin- hrs [79], MGCD0103- 9 h [80] and undergo hepatic metabolism either via the CYP450 system (romidepsin) [81] or the glucoronidation system (vorinostat) [82]. The metabolites are excreted through the biliary and fecal routes. Romidepsin is highly protein bound in plasma (92% to 94%) over the concentration range of 50 ng/mL to 1,000 ng/mL with α1-acid-glycoprotein (AAG) being the principal binding protein.
Table 2.
Classification of histone deacetyalse inhibitors
Table 3.
Listing of single agent hdacicurrently in clinical trials for lymphoproliferative disorders
| Class | Agent | Isotype selectivity | Formulation | State of clinical development in lymphoid malignancies |
|---|---|---|---|---|
| Hydroxamic acid | Vorinostat | I,2a,2b,4 | Oral, IV | FDA approved for CTCL |
| phase II trials for NHl and HD | ||||
| TSA | 1,2,4 | Not in clinical use | ||
| LAQ824 | 1,2,4 | Phase 1 studies | ||
| LBH589 (Panobinostat) | 1,2a,2b,4 | Oral | Phase II studies | |
| Pursuing approval I track in Hodgkin lymphoma | ||||
| PXD101 (Bellinostat) | 1,2a,2b,4 | Oral, IV | Phase 1 studies in NHL | |
| Phase II studies in PTCL, | ||||
| Pursuing approval track in PTCL | ||||
| ITF2357 [132] | 1,2,4 | Oral | Phase II studies in relapsed and refractory Hodgkin lymphoma- | |
| CRA-024781 | 1,2b | Oral | Phase 1 studies | |
| SB 939 | Unknown | Oral | Phase 1 studies | |
| Short chain fatty acids | Sodium butyrate | 1,2a | Not in clinical use | |
| Valproic acid | 1,2a | Oral | Case reports | |
| Combination trials | ||||
| AN-9 | NA | Phase II trials | ||
| Bezamides | SNDX-275 [133] | 1,2,3,9 | Oral | Phase II trials in Hodgkin lymphoma |
| Etinostat | ||||
| MGCD0103 | 1,2,3,11 | Oral | Phase 1 Expanded to phase II in Hodgkin lymphoma based on Impressive activity | |
| Pimelic diphenylamise | 1,2,3 | |||
| Cyclic peptides | Romidepsin | 1,2,4,6 | IV | FDA approved for CTCL |
| Possible approval for PTCL likely | ||||
| apcidin | 1,2 |
One of the original studies regarding the clinical pharmacology of these agents was reported by Kelly and O’Connor [78, 83] in their early phase experience with vorinostat. These studies were initiated with an IV formulation, and later employed an oral formulation of vorinostat. A direct comparison of the toxicity and pharmacokinetic profile of IV versus oral vorinostat revealed that the Cmax of exposure to vorinostat was substantially higher with the IV formulation vs oral formulation (2,408 ng/ml vs 658 ng/ml) and the area under the curve of exposure (AUC) was substantially greater with the oral route of exposure (4,634 hxng/ml vs 101,854 hx ng/ml). Interestingly, the toxicity profile of these two regimens was also markedly different with more thrombocytopenia, dehydration and diarrhea with the oral formulation as compared with the IV. Since this experience, there remains some debate regarding the optimal pharmacokinetic strategy to employ in administering HDAC inhibitors. For example, are these agents more efficacious with a more Cmax profile of exposure, or is daily oral dosing optimal. Of course, irrespective of these routes of administration and discrete PK issues, the half life of the individual agent will be an important determinant of dose and schedule.
Extensive pharmacokinetic monitoring in clinical trials has revealed that, romidepsin undergoes extensive metabolism in vitro primarily by CYP3A4 with minor contribution from CYP3A5, CYP1A1, CYP2B6, and CYP2C19. At therapeutic concentrations, romidepsin did not competitively inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 in vitro. Similarly oral vorinostat is 71% bound to proteins and is metabolized via glucuronidation and hydrolysis followed by beta-oxidation into two inactive metabolites. Biotransformation by cytochrome P450 is negligible. It is renally excreted with <1% of the dose recovered as unchanged drug in the urine. The mean urinary excretion of the two pharmacologically inactive metabolites at steady state was 52 ± 13.3% of vorinostat dose; 16 ± 5.8% of the dose as O-glucuronide and 36 ± 8.6% of the dose as 4-anilino-4-oxobutanoic acid of vorinostat.
In clinical trials histone acetylation seen in mononuclear cells in the peripheral blood is considered to be a biomarker for reaching target and the effect and can persist up to 10 h [78] after the drug has cleared from the system after an oral dose. Interestingly, as shown by Kelly and O’Connor [78, 83], higher doses of vorinostat did not necessarily produce more acetylated histone, but generally resulted in a longer half-life of the acetylated H3/H4. Obviously, whether this would be true for other protein targets (p52, Bcl-6, etc) remains to be determined. Despite the role of H3/H4 acetylation, it is important to recognize that accumulation of acetylated H3/H4 can be demonstrated in every patient (very similar to the proteasome inhibitor story), and that accumulation of the Ac-H3/H4 does not correlate with response. Identifying appropriate surrogate biomarkers of response with HDAC inhibitors continues to be a major pursuit.
The side effect profile of all HDAC inhibitors is fairly uniform across even the diverse chemical classes of agents. The most common side effects include fatigue, nausea, and diarrhea [1, 5, 10, 84]. Transient thrombocytopenia is the most common myelosuppressive effect. During the phase 1 experience with vorinostat, bone marrow examination of patients at their platelet nadir revealed a normocellular marrow with somewhat dysplastic appearing megakaryocytes that appeared to have impaired platelet budding [83]. This observation was also seen with LBH689 as well. Subsequent studies have revealed that HDAC inhibition may repress GATA-1 gene an important transcription factor for heamtopoeisis [85], leading to a delay in megakaryocyte maturation and thrombocytopenia [86].
One of the recurring themes of HDAC inhibitors relates to the cardiac effects of these agents. Prolongation of the QTc interval has been observed as a class effect for many HDAC inhibitors and is thought to occur via the HERGK+ channels [87, 88]. This was first noted in a 15 patient phase II romidepsin trial that had to be halted due to a fatal cardiac event and an unexpected incidence of serious cardiac arrhythmias seen in five patients (two asymptomatic nonsustained ventricular arrhythmia, three prolonged QTc interval) [89]. A subsequent systematic study of cardiac events resulting from treatment with romidepsin (282 administered cycles of Romidepsin and over 700 doses of the drug), demonstrated that almost all patients had some prolongation of the QTc interval (median 14 ms) and more than half the patients had transient EKG changes including T wave flattening and ST depression [90]. These changes were clinically insignificant with no change in cardiac enzymes or cardiac function. The agent is now approved with the caution that the treating physician should be careful in giving the drug to patients who may have a significant cardiac history or be susceptible to prolongation of the QTc interval. It is recommended that attention be paid to maintaining the patient’s potassium and magnesium levels within the normal range while receiving therapy with romidepsin. Due to the early experience with romidepsin, most trials with HDACI now require rigorous cardiac monitoring. It is also recommended that patients with baseline prolonged QTc, significant heart disease, or patients on medications that may prolong the QTc interval be excluded from trials with HDAC inhibitors. Various HDAC inhibitors have also been shown to vary in their cardiac effects. Vorinostat, for example, has not shown to be associated with any serious cardiac toxicity whereas the hydroxamic acid panobinostat (LBH589) and its predecessor LAQ824 are associated with QTc interval prolongation (one patient had tosrdaes de pointes) [91, 92]. Encouragingly, there were no reported long term changes in the EKG seen in any of the patients treated on trials of HDAC inhibitors. Thrombosis and pulmonary embolism are other important adverse events noted on the pivotal vorinostat trials (two patients experienced thrombosis and pulmonary embolism) [93]. It was unclear if this was directly related to the drug, but it is recommended that caution be used while using vorinostat in patients who may have an underlying thromboembolic disorder, an increased incidence of thromboembolic events has not been reported on the ongoing clinical trials with HDACI.
Clinical activity of HDAC inhibitors in lymphoproliferative malignancies
B-cell lymphomas
Lymphomas are the most common hematological malignancy in the United States and constitute about 5.4% of all cancers with a yearly estimated incidence of 66,000 cases of NHL and 5,000 cases of HL per year [94]. B-cell NHL are the most common type of lymphomas and include subtypes that vary from some of the most aggressive tumors known to science (Burkitt’s lymphoma) to some of the most indolent (follicular and small lymphocytic). The lymphomas are readily classified into aggressive subtypes, for which urgent chemotherapy is required to affect cure, or indolent diseases, which are more typically managed as chronic diseases. As a result, treatment is often tailored to the aggressiveness of the disease, with dose intense combination therapy and possibly autologous bone marrow transplant being considered for aggressive histologies like with Burkett’s lymphoma, mantle cell lymphoma and diffuse large B- and T-cell lymphoma, while single agent rituximab and less dose intense chemotherapies are routinely employed for the more indolent subtypes of disease.
With this background, the development of new drugs for the treatment of lymphoma can be viewed as falling into one of several distinct categories: (1) simple single agent regimens that have a role in managing relapsed or refractory diseases (ex: bortezomib, pralatrexate for PTCL, vorinostat and romidepsin for CTCL, rituximab for indolent lymphomas); (2) integration of an active agent into an existing regimen to exploit possible synergistic effects (ex: rituximab plus CHOP or EPOCH); (3) use of the agent as a maintenance therapy following definitive chemotherapy (ex: rituximab, trials of lenalidomide in mantle cell lymphoma). In this context, each and every one of these opportunities exists across the many disease sub-types of NHL. Hence, there is a need to develop novel therapies for patients with NHL to continue to improve outcomes, which have clearly improved dramatically over the past decade. HDAC inhibitors, given their reasonable side effect profile and compelling effects on molecular pathways that may be important in lymphomagenesis, clearly have the potential to impact all these major areas of drug development. Presently, there are no HDACI agents that are approved specifically for the treatment of B-cell lymphomas, though there is data with select inhibitors, and a strong rationale remains intact for their use in B-cell malignancies. Mechanistically one disease where there appears to be a strong rationale, though with minimal clinical data to date, is in mantle cell lymphoma. This unique disease is grossly characterized by marked dysregulation of cyclin D1 mediated by the t(11:14) translocation, and loss of the cyclin dependent kinase inhibitors (cdk) p21 and p27. Interestingly, two of the prominent effects of HDAC inhibitors is down-regulation of cyclin D1, and up-regulation of p21/p27. The idea that one drug could potentially revert these pathognomonic molecular features represents a promising venue for thinking about how to rationally employ such agents for such a challenging disease. Similarly, the association of dysregulated BCL-6 in many cases of DLBCL and the effects of HDACI on BCL6 as described in the section above can potentially provide a rationale for the therapeutic opportunity.
Perhaps one of the earliest demonstrations of activity of an HDAC inhibitor in B-cell lymphoma was with one of the oldest HDAC inhibitors used in medicine, namely valproic acid (Depakote). Valproic acid, approved for the treatment of seizure disorders, induced a complete remission in a patient with multiply relapsed transformed follicular lymphoma, and was one of the first published reports of the efficacy of HDAC inhibitor in the treatment of B cell malignancies [95]. Valproic acid, an acknowledged though weak inhibitor of histone deacetylases, has been more widely studied in myeloid leukemias, with little to no other experiences in lymphoid malignancies.
Beyond valproic acid, there have been several early phase trials with a variety of other HDAC inhibitors for the treatment of B-cell NHL. Kelly and O Connor [78] reported on the first phase 1 study of intravenously administered vorinostat (previously known as suberoylanidie hydroxamic acid, or SAHA) in patients with solid tumors and hematological malignancies. Of the 11 patients with B-cell malignancies (HL n = 5, NHL n = 6 ), no patients with B-cell NHL responded, though antitumor activity was seen in two patients with HD including a 30% reduction of tumor burden in one patient which was maintained for 3 months and stable disease in another patient for 8 months. A follow-up Phase 1 study conducted by the same investigators [83] with an oral formulation of vorinostat revealed a very favorable side-effect profile, and a maximum tolerated dose of 400 mg per a day given for 14 consecutive days on an every 21 day cycle. This study enrolled a total of 25 patients with hematological malignancies, with the best response being a complete remission (CR) seen in a patient with transformed follicular lymphoma. Partial responses (PRs) were seen in patients with transformed lymphoma and mycosis fungoides. A patient with Hodgkin’s disease had a 31% decrease in disease lasting for 10 months. These two studies of vorinostat in patients with solid tumor and hematologic malignancies were among the first to demonstrate the potential activity of these compounds in B-cell malignancies and Hodgkin lymphoma. Another Phase 1 trial of oral vorinostat has been conducted in patients with B-cell malignancies, as recently reported by investigators in Japan. This trial enrolled ten patients with B cell lymphomas at two dosing levels: 100 mg and 200 mg bid for 14/21 days. Four of the ten patients responded, including two CRs and one PR seen in patients with follicular lymphoma (FL) and one PR in a patient with MCL [96]. A dedicated phase 2 study of vorinostat in patients with relapsed DLBCL [97] at a dose of 300 mg bid (14 days per 3 weeks or 3 days per week) has been reported. Eighteen patients were enrolled, with one patient obtaining a CR lasting 468 days, and one patient who attained stable disease for 301 days. Based on this study, and the collective experience from the Phase 1 studies, it was concluded that vorinostat did not exhibit impressive single agent activity in large B-cell lymphoma. However, it should be noted, that the MTD now part of the label for vorinostat, was not used to assess response in these studies, and in fact, based on the phase 1 experience, as well as data generated by Duvic et al. in CTCL, this particular dose was found to be poorly tolerated. A second phase II study evaluated vorinostat at a dose of 200 mg bid for 14/21 days in patients with relapsed and refractory indolent NHL. This trial enrolled 33 out of which four patients achieved a CR, two attained a PR and 4 had stable disease [98]. These experiences seem to suggest that while the single agent activity of vorinostat in DLBCL is variable, and probably low, it raises two question: (1) why do select patients can achieve CR, while other don’t; and (2) what is the optimal pharmacokinetic profile.
The experience with vorinostat has prompted further evaluation of other more potent HDAC inhibitors for the treatment of lymphomas. Parallel to the development of these agents in myeloid malignancies, most phase I trials are conducted to include the broad category of lymphoid diagnoses that initially encompass both B- and T-cells with the idea of expanding the trial to a specific subtype if a positive signal is seen in a specific diagnostic group. Belinostat, another HDAC inhibitor that belongs to the class of hydroxamic acids is currently in development for the treatment of lymphoma, in particular peripheral T-cell lymphoma (PTCL). Similar to vorinostat, it has an oral and IV formulation, though the majority of data collected to date is with the IV formulation. The initial phase I trial of belinostat in advanced solid tumors established a maximal tolerated dose (MTD) of 1,000 mg/m2 given IV for 5 days as a 30 min infusion. This dose was confirmed as the MTD in a parallel study of the same agent in hematological malignancies with no additional toxicity seen in this group of patients. This study enrolled 11 patients (DLBCL n = 7, transformed CLL n = 2, transformed FL n = 4, CLL n = 2). No objective responses were seen in any patients though some patients experienced stabilization of disease (DLBCL n = 2, CLL n = 3). For ease of administration, the oral formulation of belinostat is being developed and a Phase 1 study of oral belinostat in patients with all sub-types of NHL is underway. The starting dose was 750 mg/m2 with planned escalations until MTD is reached. Interim results on nine patients were reported at ASCO 2009 [99]. Tumor shrinkage in the range of 43% to 49% was reported in one patient with MCL and two patients with Hodgkin lymphoma. Accrual continues as MTD has not been reached with present cohorts being treated at doses well above the MTD reached with the IV formulation again alluding to the differing properties of oral and IV forms of these agents.
Another orally administered HDAC inhibitor PCI-2478, belonging to the class of hydroxamic acid is being developed for the treatment of lymphomas. Evans et al have reported the results of the initial phase I data at ASH 2010. One patient with follicular lymphoma achieved a CR [100] while four PRs and seven SD responses were noted in 20 evaluable patients. This study has shown that there are no cardiac effects or prolongation of the corrected QT interval (QTc) interval noted with this agent. A phase II portion of the trial is planned. Several other HDACI in development for various lymphoid malignancies are listed in Table 3.
While the experience with HDAC inhibitors as single agents in B-cell lymphoma has been less than hoped for, it is clear these drugs have important potential activity, and that the likely path forward will involve rational combinations. In addition, the experience with all the available HDAC inhibitors is not consistent, as some have minimal to no data in B-cell malignancies. It will be important to gain a better understanding of how the various classes of HDAC inhibitors work in B-cell lymphoma, and to try and develop some reasonable hypotheses around how these agents are likely working in these DLBCL. With this experience intact, the emergence of potentially important HDAC inhibitor combinations will emerge. As we will discuss below, understanding the molecular pharmacology in the context of understanding the molecular pathogenesis also highlights logical drug: drug combinations likely to be synergistic in particular disease settings.
Hodgkin lymphoma
Hodgkin’s lymphoma (HL) carries a cure rate of over 85% with either primary therapy or salvage therapy with high dose chemotherapy and stem cell transplantation. Patients who fail these first and second line regimens typically have a poor prognosis with a median survival of 3 years [101]. While there appear to be many drug emerging for the treatment of NHL, it has been less obvious how any of these agents might perform in patients with relapsed or refractory Hodgkin lymphoma. To date, several HDAC inhibitors have shown activity in relapsed HL, and after T-cell lymphoma, it is the one disease venue where the clinical data is compelling enough to now entice some sponsors to pursue registration directed studies in HL.
Many lines of in vitro data support the potential activity of several HDACI against HL cell lines. HL is characterized by a high level of cytokine secretion from the inflammatory infiltrate surrounding the pathognomic Reed Sternberg (RS) cells. These cytokines include, IL-5, 6,7,9,10,13 and thymus activation-regulated chemokine (TARC/CCL17). These cytokines, many of which are part of an autocrine loop are thought to function by activating the JAK/STAT pathways, resulting in continual activation of the STAT family of transcription proteins. In particular, STAT3 and STAT6 are known to be frequently activated in HL. STAT3 activation is induced by several cytokines including IL-2, 6, 7, 9, 10,15 , while STAT6 is induced primarily by IL4 and IL13—and may be dependent on an autocrine IL-13 loop secreted by Reed Sternberg cells [102]. Phosphorylated STAT6 localizes to the nucleus and induces the expression of STAT6 target genes that include TARC and IL-13 as well as other cytokines that attract lymphocytes that belong to the Th2 class into the tumor microenvironment [103–105]. These lymphocytes are involved in humoral immunity and promote allergic responses. This suggests the importance of STAT 6 in the survival of Reed Sternberg cells as well as promoting the unique cellular and immunologic milieu that is a hallmark of HD. Interestingly, STAT regulation involves phosphorylation as well as lysine acetylation, which implies that HDAC inhibitors could play a role in regulating critical features of the HL biology [106]. This is supported by the following evidence. Buglio et al [107] have demonstrated that exposure of HL cells (L-428 and KM-H2) to vorinostat showed an increase in histone acetylation and p21 expression, and caspase mediated apoptosis. Vorinostat selectively inhibited STAT6 phosphorylation and was shown to result in decreased mRNA levels of STAT6 by PCR and a reduction of TARC as a downstream effect. Changes in cytokines were evaluated in the supernatants of exposed cells which demonstrated an increase in the level of IL13 and IP-10 (interferon inducible protein), and a significant decrease in the level of IL-5, confirming a shift in the Th1/Th2 cytokine balance. Another target gene regulated by STAT6 is the antiapoptotic Bcl-xL, which was shown to be significantly decreased in the HL cell lines following vorinostat exposure. This reduced level of Bcl-xl could alone reduce the apoptotic threshold sufficient enough to allow for rational combinations with other HL active agents. In addition to these effects on growth and survival pathways, it is also clear that HDAC inhibitors, either alone or in combination with a hypomethylating agent, can also influence antitumor immune responses by affecting the expression of proteins like the cancer testis antigens (CTA), which include MAGE, SSX and NY-ESO, in a variety of tumors including HL [108]. These immunomodulatory effects may also contribute to the therapeutic activity of these agents in HL.
Clinically, there are now a several studies that seem to consistently demonstrate that HDAC inhibitors have activity in Hodgkin’s lymphoma. One of the earliest insights into this signal was revealed in the phase 1 experience with vorinostat. In the IV and oral phase 1 experiences with vorinostat, 12 patients with relapsed or refractory HD were treated with escalating doses of vorinostat, with four patients exhibiting responses as follows: On the IV study, one patient attained a PR that lasted for approximately 9 months, one patient experienced a 14% decrease in her lung disease resulting in significant improvement of her performance status, and a third patient achieved a 42% reduction of the tumor lasting for 2 months. On the oral study, one patient achieved a 31% decrease in tumor lasting nearly 10 months A subsequent phase II trial of vorinostat administered at 200 mg orally given twice a day for 14 of 21 days produced only modest clinical activity, with only one patient achieving a PR [109]. It should be appreciated that the phase 1 experience with oral vorinostat established a MTD of 400 mg given orally once a day 14 of 21 consecutive days. This in fact is the recommended dose and schedule in the label of vorinostat for use in patients with relapsed or refractory CTCL. In fact, as we will see shortly, a well performed systematic analysis of different doses and schedules of vorinostat in CTCL clearly reaffirmed this MTD, and also provided data that alternative doses and schedules were less likely to produce clinical activity. Most, if not all phase 2 experiences with vorinostat in NHL, beyond CTCL of course, have been performed with alternative doses and schedules of vorinostat, raising an important issue: phase 2 studies employing schedules other than 400 mg PO daily may be using suboptimal dosing schedules, and thus, poor evidence in these phase 2 studies could reasonably be attributed not merely to the ‘activity’ of vorinostat in that context, but rather, to the dosing schedule. It will be important in future studies of vorinostat to employ at least the recommended phase 2 dose and schedule when exploring activity in different disease scenarios.
Of the available HDAC inhibitors, panobinostat is being studied in a registration directed phase 2 trial in Hodgkin lymphoma. The initial phase IA/II trial of panobinostat (LBH589) employed 2 different dose levels and schedules of this agent in patients with hematological malignancies. Patients with HL were entered onto the study at two dose levels: Arm 1 was dosed at a starting dose of 30 mg a day given Monday, Wednesday and Friday (MWF) every week, while arm 2 was initiated at 45 mg a day given on the same MWF every other week schedule. There were 13 patients with HL that were enrolled on the study, of which five met criteria for a partial response indicating evidence of activity HL. It appeared to be well tolerated with fatigue, nausea, thrombocytopenia and diarrhea being the most common side effects. The MTD was estimated by the logistic regression model and was defined as 40 mg a day given every MWF on the weekly schedule [110, 111]. Based on this data, a large international phase II trial of panobinostat is designed for patients with relapsed and refractory HL. While the study is now on going interim results were reported on 81 patients at ASCO 2010 with responses seen in 17 patients, two patients attaining a CR and 15 patients attaining a PR, despite the fact that the study population appears to be very heavily treated [112, 113].
Another HDAC inhibitor with activity in Hodgkin’s lymphoma is MGCD0103, which belongs to the benzamide class. MGCD 0103 is classified as an isotype selective HDAC inhibitor that predominantly inhibits class 1 HDAC enzymes and is administered as an oral formulation. A phase II trial of MGCD0103 was conducted at a dose level of 110 mg given orally three times per week in patients with relapsed and refractory HL. Responses were seen in seven of the 20 patients that were treated at this dose level including complete remissions; however the dose was considered too toxic, requiring frequent interruptions and dose reductions. The protocol was then revised to lower the dose to 85 mg per day give on the same schedule. Another ten patients were enrolled on the lower dose and partial responses were seen in three of the ten patients. The agent was well tolerated with the main side effects being fatigue and thrombocytopenia, though two patients developed pericardial effusions requiring an interruption of the protocol [114]. Collectively, these data suggest that there is a signal of efficacy of some of the more potent HDAC inhibitors in HL but the biological correlates of this activity are still unclear.
T cell lymphomas
Mature T-cell non-Hodgkin Lymphoma and NK-cell neoplasm’s comprise about 12% of all NHL and 15–20% of aggressive lymphomas worldwide. They are characterized by great morphological diversity and genetic variation even within individual disease entities. The current 2008 WHO classification recognizes over 20 types of mature T-cell and NK T-cell lymphomas (PTCL) [115]. Cutaneous T cell lymphomas (CTCL) are malignancies that arise in the skin and are classified as a separate entity based on their distinct clinical behavior and prognosis [116]. Among the aggressive lymphomas, a T-cell phenotype confers a worse clinical outcome compared to their B-cell counterparts, with the exception of ALK-positive ALCL. Long-term survival at 5 years remains at 10–30% for most histologies with present treatment strategies [117] and relapsed and refractory disease remains a significant clinical dilemma.
For reasons that are not entirely clear, HDAC inhibitors have shown relatively consistent and promising activity in the treatment of many types of T-cell lymphoma. In fact, two agents of this class, vorinostat (Zolinza) and romidepsin (Istodax) have been approved for the treatment of relapsed or refractory CTCL in the US. In addition, romidepsin, belinostat and panobinostat are all in clinical trials for T-cell malignancies, including peripheral T-cell lymphoma.
From a mechanistic perspective, it has been difficult to assign a precise mode of action of this class of drugs to any lymphoma, let alone CTCL or PTCL. It is not even clear if histone acetylation is essential for the biological activity seen in T-cell lymphomas. Pharmacodynamic studies have shown that histone acetylation can be demonstrated in peripheral blood mononuclear cells as well as [83] tumor tissue from patients with T-cell lymphoma following treatment with HDAC inhibitors, however, there is no correlation with clinical response to these agents [83]. Essentially every patient achieves acetylation of histone. Duvic et al. [118] have attempted to look at biological correlatives of HDAC inhibitor therapy in patients with CTCL by performing serial skin biopsies on patients receiving vorinostat on trial at 2 h, and then at 4, 8 and 12 weeks after initiation of treatment. These results established the following: at 4 weeks, 39% of the patient samples demonstrated lymphocyte depletion consistent with the anti-lymphoma effect of vorinostat. Samples were also studied for acetylated histones at baseline and then at 4 weeks post therapy. Increased acetylation within lymphocytes post-therapy could not be demonstrated or correlated with response as most of the lymphocytes were not present after treatment. At 4 weeks post-therapy, there was a decrease in dermal microvessel density as measured by CD31 positivity on dermal vessels in all patients which was significantly lower in responding patients (p = 0.001). Prior cell line data using the CTCL cell line HH indicated that a 24 h exposure to vorinostat resulted in an 8-fold increase of the antiangiogenic protein TSP-1 as studied by gene expression array, Consistent with the cell line data, an increase in the dermal TSP-1 staining was noted at 2 h in four out of eight patient samples including two responders, and at 8 weeks a dermal increase in TSP-1 was demonstrated in six of the 17 paired lesions including four of the six responders. Another important protein that is constitutively activated in CTCL is p-STAT3, which can be detected by immunohistochemical stains either in the nucleus or cytoplasm within both the keratinocytes and the lymphocytes present within lesions. In this study, nuclear staining for p-STAT-3 was prominent in both keratinocytes and lymphocytes prior to the start of therapy. After 4 weeks of therapy with vorinostat, the staining pattern shifted to localization within the cytoplasm in nine of the 11 patients who responded, where as this shift was noted in only three of the 16 non-responders. This shift was noted as early as 2 h after treatment in four of 11 paired lesions [59].
Using a similar model of paired skin biopsy, Prince et al performed gene expression profiles (GEP) and real time quantitative PCR on skin samples from six patients with cutaneous T cell lymphoma who were being treated with panobinostat in a phase I trial. These biopsies were obtained at 0, 4, 8 and 24 h after administration of drug. In this study there were ten patients with a diagnosis of relapsed CTCL who were treated at varying dose levels as part of a large phase I study in patients with hematological malignancies. One patient received drug at 30 mg a day at the MWF weekly schedule and the remaining nine patients received panobinostat at 20 mg a day on MWF weekly. Clinical efficacy was observed in eight patients (two achieved a CR at both dose level and four patients achieved a PR, two patients had stable disease (SD)). The skin biopsy data demonstrated that there was hyperacetylation of histone H3 observed in tumor cells as early as 4 h after treatment. Consistent with previous data, histone acetylation within mononuclear cells was demonstrated in both responders and non-responders up to 48 to 72 h after the last oral dose indicating that this could not be used as a therapeutic marker. GEP data from all six patients consistently showed that panobinostat induced transcriptional regression of a greater number of genes than activation. Further statistical analysis indicated that 20 genes were consistently repressed and three genes were consistently activated following treatment with panobinostat. The genes that were consistently affected included genes affecting cell cycle (CCNDI, IGFI) apoptosis (septin10, TEF, SORBBS2), angiogenesis (GUCY1A1, ANGPT1) and immune modulation (LAIR1). Interestingly, CDKN1A, which codes for p21, has been shown to be consistently unregulated in response to HDAC inhibitor therapy, though in this study upregulation of p21 was not consistently seen in all patients. Of the 23 genes, 4 were further selected for validation by QRT-PCR. These data confirmed downregulation of guanylate cyclase 1A3 (GUCY1A3), the proangiogenic gene ANGPT1a and the transcription factor COUP-TFII (NR2F20 which is an upstream regulator of ANGPT1 and CCND1). This affect on genes controlling angiogenesis is consistent with the effects noted by Duvic et al. [59] {as discussed above and provides confirmation that the anti-angiogenic affects of HDAC inhibitor therapy may be important in their mechanism of action. Bates et al. [119] have conducted a clinical trial of romidepsin in patients with PTCL and CTCL and have also looked at the biological correlates of activity of HDAC inhibitor therapy. Predetermined markers of HDAC inhibitor activity including global histone acetylation were studied in peripheral blood sample. In addition tumor samples were collected to look for ABCB1 gene (encodes for the p-glycoprotein MDR) expression and an increase in the level of fetal hemoglobin as these genes were known to be modified by HDAC inhibitors therapy. The histone acetylation data correlated with PK parameters of AUC and C max, though there was no correlation between response, histone acetylation and the expression of either ABCB1 or fetal hemoglobin.
Increasingly, T-cell malignancies are found to be associated with dysregulation of the T-cell receptor (TCR) signaling and the immune function which contributes to the clinical syndrome. Hence, it is likely that the therapeutic effects of HDACI in T-cell lymphomas may be related to their effects on TCR and the associated immune effects. Investigators have started to look at the effects of HDAC inhibitors particularly vorinostat on TCR signaling and the immune system in order to delineate a more specific mechanisms of action for this agent, as well as to understand the basis for combining it with other agents. Woznial et al. [120] have performed extensive studies using GEP on a panel of CTCL cell lines (HH, HUT78, MJ, Myla, SeAx) that were exposed to vorinostat at various time points. Their results show that as previously seen vorinostat exposure resulted in apoptosis in these cell lines in a dose dependent manner, affected cell cycle progression (G2M and G0/G1 arrest) and induced hyperacetylation of all four core histone proteins. GEP confirmed that 20% of the genes were significantly activated or repressed in response to vorinostat in 24 h. The functional analysis of these altered genes revealed pathways that have already been identified as being affected by HDAC inhibitors including cycle regulators for G1/S transition (E2F, E2F4, CDK4, CDk6, Cyclin A2, D2,D3,E20) ,G2/M regulators (CDC23, CD25B and CHEK4), apoptosis (FAS, IRAK1, CASP6, BID, BCL2), antiproliferative genes as well as multiple mitogen activated signaling kinase (MAPK) signaling pathway ( MAPK1, MAP3K6, MAP3K14) as described in the sections above. However, this study has demonstrated changes in genes that are involved in the JAK/STAT signaling pathway, cytokine–cytokine interaction and expression of receptors belonging to the tumor necrosis factor (TNF) family, all important pathways for survival and differentiation of lymphocytes and the immune system. Vorinostat treatment was shown to shift the expression profile of cytokines resulting in increased expression of interleukins like IL1a, IL6, Il9, and a decrease in the expression of IL4, IL5, Il10, IL11 and their associated receptors. Overall the cytokine profile represented a state that inhibited lymphocyte growth and proliferation and inhibited the TH2 type immune responses. This latter aspect of the drug effect is important as CTCL is a malignancy of activated T cells and is characterized by dysregulation of the immune system with reversal of the Th1/Th2 cytokine profile [121]. Vorinostat has also been shown to affect genes that affect cell migration and chemotaxis that may affect the skin homing properties of malignant cells in CTCL. While it is difficult to list all the genes that were altered, overall there was a decrease in the expression of certain cytokine genes like CCL1, CCL22, CXCL10, CCR4 and CCR6 and an increase in others like CCR2, CCR6. There were also some alteration in the expression of members of the JAK/STAT pathway themselves like STAT6, STAT5A, SOCS2, (decrease) and STAT1, STAT3, JAK1 (increase).
Another important pathway required for T-cell survival and proliferation involves the T cell receptor (TCR). TCR signaling induces activation of many protein tyrosine kinases resulting in phosphorylation of many downstream substrates including CD3 chains, TCR epsilon chain, and the zeta chain associated with ZAP-70 and phospholipase C [122]. After antigen stimulation, TCR activation leads to activation of LCK and FYN which results in tyrosine phosphorylation, after which ZAP-70 tyrosine kinase is recruited to the TCR where it acts together with LCK to activate downstream substrates including PKC, and MAPK as well as the Jun pathway. In addition, it is linked to the PI3K/AKT pathway which is important for the survival of T-lymphocytes through an increased expression of BCL-XL. Treatment with vorinostat induces repression of all genes associated with TCR related signaling including ZAP70, CD3DIL4, IL5, Il10, FOXP3 and upregulated FYN, IFNG and IL12A. The effects on TCR signaling were significant and were seen across all cell lines and confirmed by QRT-PCR. A decrease in the phosphorylated forms of ZAP-70 and AKT after vorinostat treatment was confirmed by western blots confirming the inhibitory effects of this agent on TCR signaling. FYN which seems to be upregulated by vorinostat functions is a SRC family kinase that phosphorylates several negative regulators of TCR signaling and ultimately adds to the negative effect of vorinostat on TCR signaling pathways. These changes were seen in cell lines and it is possible that there may be differences in gene expression that may be seen in actual tissue samples, and variability associated with the various subtypes of HDACI.
Clinically, there are many HDAC inhibitors that are being studied for the treatment of T-cell lymphoproliferative malignancies. While each has its own strengths and limitations, we will briefly review those HDAC inhibitors presently in clinical study for T-cell NHL.
Vorinostat (formerly known as suberoylanilide hydroxamic acid—SAHA) was the first HDAC inhibitor approved for the treatment of cancer. In the US this agent is approved for the treatment of CTCL in patients who have failed at least two prior systemic therapies [1]. Based on early signals of activity and a favorable toxicity profile in patients with lymphoma in the phase 1 experience, Duvic et al. [59] conducted a restricted phase I/II study of oral Vorinostat in patients with CTCL. This Phase 2 experience enrolled 33 patients with advanced heavily pretreated CTCL patients. Three different orally administered dosages and schedules were sequentially evaluated: 400 mg daily; 300 mg twice daily for 3 days followed by 4 days of rest; and 300 mg twice daily for 14 days with a week of rest followed by 200 mg twice daily. The overall response rate (ORR) was 24.4% with eight patients having a partial remission (PR) including four patients with Sezary syndrome. More importantly, 14 of the 33 patients (42%) reported significant pruritis relief. The median time to response was 11.9 weeks, and the median duration of response was 15.1 weeks. The major side effects included fatigue, thrombocytopenia, and gastrointestinal symptoms (predominantly diarrhea and nausea). Within the different cohorts, more thrombocytopenia was noted in the third cohort where patients received a higher dose of 300 mg bid for 14 days straight. However, most of the responses were seen in Groups 1 and 3. Thus, based on the response rate and toxicity criteria, the 400 mg per day dose was established as having the best safety profile. This study affirmed the findings in the original phase 1 experience, supporting the 400 mg PO daily dosing of vorinostat, which was associated with the most favorable side effect profile and highest response rate of any of the explored schedules. It is for this reason that conclusions about the efficacy of vorinostat with other doses and schedules may be misleading if the phase 2 study was not conducted at the recommended Phase 2 dose based on the Phase 1 experiences. Based on these data, a registration directed phase II study using the 400 mg PO dose was initiated by Olsen et al. [93]. This study included 74 pts with CTCL who had failed at least two prior systemic therapies. Disease assessment was done by modified skin weighted assessment tools (m-SWAT). This study demonstrated an overall response rate (ORR) of 29.7%, with one patient achieving CR after 281 days of therapy. Median time to objective response was 56 days (28–171) though some patients took up to 6 months to respond and the median duration of response was not reached in the study though it was greater than 185 days. Non-progressing patients continued on the study with 15 patients receiving drug for over a year and six patients who received it for more than 2 years. The response to vorinostat was not impacted by their response to last systemic therapy. In addition, another 29 patients had clinical benefit manifested by stable disease for over 24 weeks. Prurtis relief was seen in 305 of patients including responders and non-responders again pointing to the effect of vorinostat on cytokine profiles. These data supported the approval of vorinostat by the U.S. FDA in October of 2006, making it the first HDAC inhibitor ever approved for the treatment of cancer.
Romidepsin (formerly referred to as depsipeptide, FK228) is cyclic peptide originally isolated from the broth culture of Chromobacterium violaceum. It was initially studied at the National Cancer Institute in patients with refractory or relapsed solid tumors by Bates and Piekarz. Piekarz et al reported a case of patient with refractory PTCL that responded to depsipeptide [123]. As the drug began to demonstrate a consistent signal in patients with T-cell lymphoma, the study was expanded to separate out patient with CTCL from those with PTCL, and then further stratified patients based on the amount of prior therapy they received. Overall it had a stratification that consisted of seven different treatment arms with the intention of separating out different cohorts of patients with T-cell lymphomas based on histology and prior number of therapies. All patients received drug as an intravenous infusion at a dose of 14 mg/m2 once a week in a 23 out of a 4 week cycle. The two major cohorts were CTCL and PTCL and the data from these two groups of patients has been analyzed and presented separately. Piekarz et al reported the results of 71 patients with relapsed CTCL that were treated with depsipeptide. The overall response rate was 34% (24/71) with complete responses observed in four patients. The median duration of response was 13.7 months. Based on the data generated by the NCI, the drug was eventually acquired by Gloucester Pharmaceuticals, and two registration directed clinical trials were launched, one in CTCL and one in PTCL. In the preparation of the New Drug Application to the U.S. FDA, data from the two studies (NCI plus Gloucester) was pooled and patients were analyzed using a composite end point to assess response using skin assessment, lymph node and visceral involvement and abnormal circulating Sezary cells. One-hundred thirty five patients were evaluable with a median age of 57 years who had received a median of at least 2 (1–8) prior systemic therapies. One hundred three patients (76%) had at least stage IIB or higher disease and 19 patients had SS. The overall response rate (ORR) was 41% with a complete response (CR) rate of 7% and duration of response of 14.9 months. Interestingly, the response rate (RR) was 58% in patients with Sezary Syndrome. Unique to the romidepsin study was a specific symptom assessment tool to quantify changes in pruritis. In this study a relief of pruritis was seen in over 60% of the patients. This agent was approved by the FDA for the treatment of relapsed CTCL following failure of one line of systemic therapy. This trial included cohorts of patients with PTCL who had failed at least one prior systemic therapy. The NCI sponsored study with 48 evaluable patients showed an overall response rate of 31% with 4 (8%) CRs. Patients who got more than 2 cycles of therapy had a response rate of 44%. Median Duration of response was 9 months with a median time to progression of 12 months. The results of the Gloucester sponsored study of romidepsin in patients with PTCL are still awaited.
Belinostat is a hydroxamic acid with pan HDACI activity that is currently in clinical trials for solid tumors and hematological malignancies available in both oral and intravenous formulation. A Phase I trial of the intravenous formulation was carried out in parallel to the solid tumor phase and an MTD of 1,000 mg/m2 was established. This formulation is being studied in a large phase II trial at a dose of 1,000 mg/m2 for patients with relapsed PTCL. At the same time the oral formulation has been developed and similar to the story with vorinostat has been shown to be better tolerated than the IV formulation. In the ongoing phase I trial of belinostat in hematological malignancies, the dose is up to 2,000 mg/m2 and an MTD is not reached [124].
Oral Panobinostat, also known as LBH589, is a pan HDAC inhibitor belonging to the hydroxamic acid group that has shown remarkable activity in patients with CTCL in a phase I/II trial. Six out of ten patients with CTCL showed a response in the original phase 1 trial at a dose of 20 mg and 30 mg MWF on a weekly basis. The trial has now been expanded to a dedicated phase II in patients with CTCL at a dose of 20 mg a day MWF given weekly. The results of this trial are awaited.
Combinations of HDAC inhibitors with other agents
As noted above, there is ample evidence for the single agent activity of HDAC inhibitors as anti lymphoma agents. By targeting specific molecular pathways they also lend themselves to endless combinations with other targeted therapies. Some of the more promising combinations and the mechanistic rationale behind them is discussed below.
Combinations with proteasome inhibitors (PI)
Proteosome inhibitors (PI) act by targeting the catalytic 20S core of the proteaosome and induce apoptosis in cancer cells by several mechanisms. They have emerged as a new class of therapeutic agents that are effective in the treatment of several malignancies including MCL, and T cell lymphomas. Combinations of HDAC inhibitors and the PI bortezomib show marked synergistic clinical activity in some lymphoproliferative disorders like multiple myeloma and mantle cell lymphoma. Paoluzzi et al. [125] have demonstrated synergistic cytotoxicty of either belinostat or romidepsin in combination with boretezomib against a panel of MCL cell lines. The combination of HDAC inhibitors and PI was shown to induce mitochondrial membrane depolarization and apoptosis in treated cells. A decrease in Cyclin D and BcL-xL was also demonstrated. The combination of vorinostat and bortezomib has been evaluated in vitro for synergy against CTCL cell lines as well. This combination has demonstrated an upregulation of p21 and p27, an increased expression of phosphorylated p38, which participates in signaling cascade-controlling cellular responses to cytokines and stress. Decreased expression of the VEGF was also demonstrated after treatment with vorinostat and bortezomib. Combinations of vorinostat and boretezomib were also synergistic in MCL cell lines resulting in apoptosis, ROS generation, and decreased NK-kB activity [126]. Similar synergy between romidepsin and belinostat with boretezomib has also been reported in CLL cell lines and has been shown to involve mechanisms of NF-Kappa B inactivation, and perturbation of the apoptotic pathways [127]. The novel PI carfilzomab is also being evaluated in combinations with HDAC inhibitors and has shown promising synergistic activity with vorinostat [128] in DLBCL cell line. This was associated with activation of JNK, p38 MAPK, and decrease in NK-kB, AKT inactivation and Ku70 acetylation. Unexpected synergy was also noted between the antihistamine cyproheptidine and boretezomib in MCL cell lines. Cyproheptidine is shown to be an HDAC inhibitor that resulted in and decreased Cyclin D expression—a hallmark of MCL. When combined with boretezomib , the combination was markedly synergistic in terms of cytotoxicity and was shown to result in apoptosis of cells [129]. Clinical trials are now underway to evaluate the combination of proteosome inhibitors and HDAC inhibitors for the treatment of lymphomas.
Combinations targeting the BCL-6—p53 pathway
Bcl6 is over-expressed in several B cell malignancies particularly DLBCL and other germinal cell tumors due to mutations of its genomic locus [50]. In the deacetylated state, Bcl6 functions as a transcriptional co-repressor of several genes that influence cell cycling and the tumor suppressor p53 activity. Sirtuin2- alpha, a class III HDAC, has been shown to interact and attenuate p53 mediated function [57]. While, the BCL-6 can be acetylated and activated by HDAC inhibitors, nicotinamide (vitamin B3) functions as an inhibitor of NAD-dependent deacetylation and enhances p53 acetylation and activation. Recent studies in DLBCL transcriptional signatures have revealed a subset of DLBCLs that are particularly dependent on BCL6 to maintain their survival and these patients could be candidates for clinical trials of BCL6 inhibitors. The use of HDAC inhibitors to acetylate and enhance the function of BCL-6 could conceivably provide a therapeutic target for antilymphoma therapy. This can be further enhanced by combining this with an agent like Nicotinamide that can enhance p53 function to target BCL-6 addicted tumors. Clinical trials are underway to study this combination.
Other combinations
By lowering the apoptotic threshold, HDAC inhibitors lend themselves as ideal agents to be combined with chemotherapeutic agents, a strategy that is being explored in solid tumors and other malignancies and will likely be explored in lymphoproliferative disorders. A phase I clinical trial in patients with advanced CTCL consists of vorinostat and escalating doses of baxarotene given daily for 28 days. At the time of reporting, 19 patients had been enrolled and accrual was ongoing as MTD had not been reached. Responses have been reported in some patients [130].
Another promising platform consists of combining Bcl-2 antagonists ABT-737 with an HDAC inhibitor that has been studied in various human myeloma and leukemia cell lines [131]. Vorinostat has been shown to increase the expression of Bim, Puma and Noxa that was largely sequestered by bcl-2 and bcl-xl. ABT-737 abrogated this interaction, thus promoting Bax/Bak activation and apoptosis. Clinical activity needs to be established in a clinical trial.
Conclusions
The use of HDAC inhibitors has allowed pharmacologic manipulation of dysregulated genes in cancer cells and have shown single agent activity against T cell lymphomas, CTCL, mantle cell lymphomas and HL. The bigger promise lies in the impact that these agents can have in enhancing the activity of other targeted therapies ranging from antifolates like pralatrexate to demethylating agents, proteosome inhibitors, anti Bcl-2 agents. By modifying the expression of specific genes and proteins like CD30, or growth receptors like EGFR it is possible that HDAC inhibitors could enhance the activity of targeted antibody therapies like SGN-35 or herceptin. In addition, the vast effects of HDAC inhibitors on the immune system and cytokines as seen in CTCL and HL also indicate that HDAC inhibitors can be useful in the treatment of immune dysfunction underlying autoimmune disorders, graft vs host disease. There is also an effort to determine if class specificity of HDAC inhibitors has a biological significance and as we get more information about epigenetic dysregulation in lymphoid diseases the clinical significance of this will become clear. It is likely that in the future an HDAC inhibitor based therapy will be the backbone of both upfront and salvage therapies for most lymphomas and will lead to better outcomes as compared to current cytotoxic therapies.
Acknowledgments
Conflict of interest statements
Jasmine Zain: Speakers bureau at Allos Therapeutics
Owen O’Connor: Research support from Merck and Gloucester
Logistical support during submission of this article was provided by Springer Healthcare LLC. This support was funded by Novartis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Jasmine Zain, Email: Jasmine.zain@nyumc.org.
Owen A. O’Connor, Email: owen.oconnor@nyumc.org
References
- 1.Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 2.Campas-Moya C. Romidepsin for the treatment of cutaneous T-cell lymphoma. Drugs Today (Barc) 2009;45:787–795. doi: 10.1358/dot.2009.45.11.1437052. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell. 2004;116:259–272. doi: 10.1016/S0092-8674(04)00044-3. [DOI] [PubMed] [Google Scholar]
- 5.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 6.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 7.Lagger G, O’Carroll D, Rembold M, et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi CM, Luo Y, Yin Z, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 9.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, Cang S, Ma Y, Petrillo RL, Liu D. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batty N, Malouf GG, Issa JP. Histone deacetylase inhibitors as anti-neoplastic agents. Cancer Lett. 2009;280:192–200. doi: 10.1016/j.canlet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 14.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 15.Marquard L, Gjerdrum LM, Christensen IJ, Jensen PB, Sehested M, Ralfkiaer E. Prognostic significance of the therapeutic targets histone deacetylase 1, 2, 6 and acetylated histone H4 in cutaneous T-cell lymphoma. Histopathology. 2008;53:267–277. doi: 10.1111/j.1365-2559.2008.03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doorn R, Zoutman WH, Dijkman R, et al. Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. J Clin Oncol. 2005;23:3886–3896. doi: 10.1200/JCO.2005.11.353. [DOI] [PubMed] [Google Scholar]
- 17.van Doorn R, Gruis NA, Willemze R, van der Velden PA, Tensen CP. Aberrant DNA methylation in cutaneous malignancies. Semin Oncol. 2005;32:479–487. doi: 10.1053/j.seminoncol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Zani VJ, Asou N, Jadayel D, et al. Molecular cloning of complex chromosomal translocation t(8;14;12)(q24.1;q32.3;q24.1) in a Burkitt lymphoma cell line defines a new gene (BCL7A) with homology to caldesmon. Blood. 1996;87:3124–3134. [PubMed] [Google Scholar]
- 19.LaForgia S, Morse B, Levy J, et al. Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci U S A. 1991;88:5036–5040. doi: 10.1073/pnas.88.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahuja N, Mohan AL, Li Q, et al. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997;57:3370–3374. [PubMed] [Google Scholar]
- 21.Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 22.Esteller M, Guo M, Moreno V, et al. Hypermethylation-associated inactivation of the cellular retinol-binding-protein 1 gene in human cancer. Cancer Res. 2002;62:5902–5905. [PubMed] [Google Scholar]
- 23.Martinez-Delgado B, Melendez B, Cuadros M, et al. Frequent inactivation of the p73 gene by abnormal methylation or LOH in non-Hodgkin’s lymphomas. Int J Cancer. 2002;102:15–19. doi: 10.1002/ijc.10618. [DOI] [PubMed] [Google Scholar]
- 24.Siu LL, Chan JK, Wong KF, Choy C, Kwong YL. Aberrant promoter CpG methylation as a molecular marker for disease monitoring in natural killer cell lymphomas. Br J Haematol. 2003;122:70–77. doi: 10.1046/j.1365-2141.2003.04396.x. [DOI] [PubMed] [Google Scholar]
- 25.Toyota M, Sasaki Y, Satoh A, et al. Epigenetic inactivation of CHFR in human tumors. Proc Natl Acad Sci U S A. 2003;100:7818–7823. doi: 10.1073/pnas.1337066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navas IC, Ortiz-Romero PL, Villuendas R, et al. p16(INK4a) gene alterations are frequent in lesions of mycosis fungoides. Am J Pathol. 2000;156:1565–1572. doi: 10.1016/S0002-9440(10)65028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarisbrick JJ, Mitchell TJ, Calonje E, Orchard G, Russell-Jones R, Whittaker SJ. Microsatellite instability is associated with hypermethylation of the hMLH1 gene and reduced gene expression in mycosis fungoides. J Invest Dermatol. 2003;121:894–901. doi: 10.1046/j.1523-1747.2003.12496.x. [DOI] [PubMed] [Google Scholar]
- 28.Scarisbrick JJ, Woolford AJ, Calonje E, et al. Frequent abnormalities of the p15 and p16 genes in mycosis fungoides and sezary syndrome. J Invest Dermatol. 2002;118:493–499. doi: 10.1046/j.0022-202x.2001.01682.x. [DOI] [PubMed] [Google Scholar]
- 29.Gloghini A, Buglio D, Khaskhely NM, et al. Expression of histone deacetylases in lymphoma: implication for the development of selective inhibitors. Br J Haematol. 2009;147:515–525. doi: 10.1111/j.1365-2141.2009.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villagra A, Cheng F, Wang HW, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villagra A, Sotomayor EM, Seto E. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene. 2010;29:157–173. doi: 10.1038/onc.2009.334. [DOI] [PubMed] [Google Scholar]
- 32.Simms-Waldrip T, Rodriguez-Gonzalez A, Lin T, Ikeda AK, Fu C, Sakamoto KM. The aggresome pathway as a target for therapy in hematologic malignancies. Mol Genet Metab. 2008;94:283–286. doi: 10.1016/j.ymgme.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fournel M, Bonfils C, Hou Y, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7:759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 35.Peart MJ, Smyth GK, van Laar RK, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- 37.Peart MJ, Tainton KM, Ruefli AA, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003;63:4460–4471. [PubMed] [Google Scholar]
- 38.Kawamata N, Chen J, Koeffler HP. Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood. 2007;110:2667–2673. doi: 10.1182/blood-2005-11-026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandor V, Senderowicz A, Mertins S, et al. P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambucetti LC, Fischer DD, Zabludoff S, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999;274:34940–34947. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- 41.Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat Rev Drug Discov. 2007;6:21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 44.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 45.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2:151–163. [PubMed] [Google Scholar]
- 46.Chen Z, Clark S, Birkeland M, et al. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) alpha and beta messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett. 2002;188:127–140. doi: 10.1016/S0304-3835(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 47.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 48.Kee BL, Rivera RR, Murre C. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-beta. Nat Immunol. 2001;2:242–247. doi: 10.1038/85303. [DOI] [PubMed] [Google Scholar]
- 49.Bereshchenko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 50.Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101:2914–2923. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 51.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/S0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel JH, Du Y, Ard PG, et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen HY, Lavu S, Bitterman KJ, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/S1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 56.Kovacs JJ, Murphy PJ, Gaillard S, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 57.Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 58.Meier C, Hoeller S, Bourgau C, et al. Recurrent numerical aberrations of JAK2 and deregulation of the JAK2-STAT cascade in lymphomas. Mod Pathol. 2009;22:476–487. doi: 10.1038/modpathol.2008.207. [DOI] [PubMed] [Google Scholar]
- 59.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2006;13:129–140. doi: 10.1038/sj.cdd.4401686. [DOI] [PubMed] [Google Scholar]
- 61.Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21:1773–1782. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- 62.Shao W, Growney JD, Feng Y, et al (2010) Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int J Cancer [DOI] [PubMed]
- 63.Guo F, Sigua C, Tao J, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004;64:2580–2589. doi: 10.1158/0008-5472.CAN-03-2629. [DOI] [PubMed] [Google Scholar]
- 64.Nebbioso A, Clarke N, Voltz E, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 65.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 66.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 67.Hofmann K. The modular nature of apoptotic signaling proteins. Cell Mol Life Sci. 1999;55:1113–1128. doi: 10.1007/s000180050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newbold A, Lindemann RK, Cluse LA, Whitecross KF, Dear AE, Johnstone RW. Characterisation of the novel apoptotic and therapeutic activities of the histone deacetylase inhibitor romidepsin. Mol Cancer Ther. 2008;7:1066–1079. doi: 10.1158/1535-7163.MCT-07-2256. [DOI] [PubMed] [Google Scholar]
- 69.Lindemann RK, Newbold A, Whitecross KF, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci U S A. 2007;104:8071–8076. doi: 10.1073/pnas.0702294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 71.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruefli AA, Ausserlechner MJ, Bernhard D, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler LM, Zhou X, Xu WS, et al. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 76.Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/S0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 77.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–18. doi: 10.1016/S1535-6108(03)00165-X. [DOI] [PubMed] [Google Scholar]
- 78.Kelly WK, Richon VM, O’Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 79.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 80.Garcia-Manero G, Assouline S, Cortes J, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lech-Maranda E, Robak E, Korycka A, Robak T. Depsipeptide (FK228) as a novel histone deacetylase inhibitor: mechanism of action and anticancer activity. Mini Rev Med Chem. 2007;7:1062–1069. doi: 10.2174/138955707782110178. [DOI] [PubMed] [Google Scholar]
- 82.Rubin EH, Agrawal NG, Friedman EJ, et al. A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res. 2006;12:7039–7045. doi: 10.1158/1078-0432.CCR-06-1802. [DOI] [PubMed] [Google Scholar]
- 83.O’Connor OA, Heaney ML, Schwartz L, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 84.(2010) Romidepsin (Istodax) for cutaneous T-cell lymphoma. Med Lett Drugs Ther 52:42–3 [PubMed]
- 85.Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsuoka H, Unami A, Fujimura T, et al. Mechanisms of HDAC inhibitor-induced thrombocytopenia. Eur J Pharmacol. 2007;571:88–96. doi: 10.1016/j.ejphar.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Bates SE, Rosing DR, Fojo T, Piekarz RL. Challenges of evaluating the cardiac effects of anticancer agents. Clin Cancer Res. 2006;12:3871–3874. doi: 10.1158/1078-0432.CCR-06-1017. [DOI] [PubMed] [Google Scholar]
- 88.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25:3362–3371. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 89.Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 90.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 91.Fischer T (2005) Resultsof caridac monitoring during pahse trils of a novel HDACinhibitor LBH589 in patients with advanced solid tumros and hematological malignancies. J Clin Oncol 23
- 92.Rowinsky EK (2005) Cardia monitoring in phase 1 trials of a nove HDACi LAQ824 in patients with adcanced solid tumor and hematologic malignancies JClin Oncol 23
- 93.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 94.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92:1240–1251. doi: 10.1093/jnci/92.15.1240. [DOI] [PubMed] [Google Scholar]
- 95.Zain J, Rotter A, Weiss L, Forman S, Kirschbaum MH. Valproic acid monotherapy leads to CR in a patient with refractory diffuse large B cell lymphoma. Leuk Lymphoma. 2007;48:1216–1218. doi: 10.1080/10428190701344907. [DOI] [PubMed] [Google Scholar]
- 96.Watanabe T, Kato H, Kobayashi Y, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crump M, Coiffier B, Jacobsen ED, et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol. 2008;19:964–969. doi: 10.1093/annonc/mdn031. [DOI] [PubMed] [Google Scholar]
- 98.Kirshbaum M (2007) Phase 2 study of SAHA in relapsed or refractory indolent non-Hodgkin lymphoma: a California Cancer Consortium Study. J Clin Oncol 25
- 99.Zain J (2009) Final results of a phase 1 study of oral belinostat (PXD101) in patients wieht lymphoma. J Ckin Oncol 27(suppl): abstract 8580
- 100.Evans A (2009) Phase 1 analysis of the safety and pharmacodynamics of the novel broad spectrum HDACi PCI-24781 in relapsed and refractory lymphoma. Blood 114: abstract 2726
- 101.Horning S (2008) Defining a population of Hodgkin lymphoma patients for novel therapeutics; an interantional effort. Ann Oncol 20
- 102.Kapp U, Yeh WC, Patterson B, et al. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med. 1999;189:1939–1946. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 104.Re D, Thomas RK, Behringer K, Diehl V. From Hodgkin disease to Hodgkin lymphoma: biologic insights and therapeutic potential. Blood. 2005;105:4553–4560. doi: 10.1182/blood-2004-12-4750. [DOI] [PubMed] [Google Scholar]
- 105.Skinnider BF, Kapp U, Mak TW. The role of interleukin 13 in classical Hodgkin lymphoma. Leuk Lymphoma. 2002;43:1203–1210. doi: 10.1080/10428190290026259. [DOI] [PubMed] [Google Scholar]
- 106.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 107.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shichijo S, Yamada A, Sagawa K, et al. Induction of MAGE genes in lymphoid cells by the demethylating agent 5-aza-2′-deoxycytidine. Jpn J Cancer Res. 1996;87:751–756. doi: 10.1111/j.1349-7006.1996.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirshbaum M (2007) Vorinostat in relapsed or refractory Hodgkin lymphoma: SWOG 0517. Blood 110: abstract 2574
- 110.Andrew Spencer MP, DeAngelo DJ, Blood (ASH Annual Meeting Abstracts) N (2007) Phase IA/II Study of Oral LBH589, a Novel Deacetylase Inhibitor (DACi), Administered on 2 Schedules, in Patients with Advanced Hematologic Malignancies. Blood (ASH Annual Meeting Abstracts) 110:A907
- 111.Dickinson M, Ritchie D, DeAngelo DJ, et al. Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin Lymphoma. Br J Haematol. 2009;147:97–101. doi: 10.1111/j.1365-2141.2009.07837.x. [DOI] [PubMed] [Google Scholar]
- 112.HM P (2007) Phase 1 study of oral LBH589, a novel DACi in advnaced solid tumors and NHL. J Clin Oncol 25
- 113.Sureda A. Interim results for the phase II study of panobinostat (LBH589) in patients (Pts) with relapsed/refractory Hodgkin’s lymphoma (HL) after autologous hematopoietic stem cell transplant (AHSCT) J Clin Oncol. 2010;28:7s. doi: 10.1200/JCO.2009.25.9937. [DOI] [Google Scholar]
- 114.Younes A (2007) isotype-selective HDAC-inhibitor MGCD0103 decreases serum TARC concentrations and produces clincial responses in heavily pretreated patients with relapsed classical HL. Blood 110
- 115.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 117.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 118.Duvic M, Vu J. Vorinostat in cutaneous T-cell lymphoma. Drugs Today (Barc) 2007;43:585–599. doi: 10.1358/dot.2007.43.9.1112980. [DOI] [PubMed] [Google Scholar]
- 119.Bates SE, Zhan Z, Steadman K, et al. Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br J Haematol. 2010;148:256–267. doi: 10.1111/j.1365-2141.2009.07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wozniak MB, Villuendas R, Bischoff JR, et al. Vorinostat interferes with the signaling transduction pathway of T-cell receptor and synergizes with phosphoinositide-3 kinase inhibitors in cutaneous T-cell lymphoma. Haematologica. 2010;95:613–621. doi: 10.3324/haematol.2009.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- 122.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 123.Piekarz RL, Robey R, Sandor V, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.V98.9.2865. [DOI] [PubMed] [Google Scholar]
- 124.Pohlman B (2009) Final results of a phase 2 trial of belinostat in patients with recurrent or refractory peripheral or cutaneous Tcell lymphomas. Blood
- 125.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O’Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16:554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 126.Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 127.Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008;14:549–558. doi: 10.1158/1078-0432.CCR-07-1934. [DOI] [PubMed] [Google Scholar]
- 128.Dasmahapatra G, Lembersky D, Kramer L, et al. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010;115:4478–4487. doi: 10.1182/blood-2009-12-257261. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Paoluzzi L, Scotto L, Marchi E, Seshan VE, O’Connor OA. The anti-histaminic cyproheptadine synergizes the antineoplastic activity of bortezomib in mantle cell lymphoma through its effects as a histone deacetylase inhibitor. Br J Haematol. 2009;146:656–659. doi: 10.1111/j.1365-2141.2009.07797.x. [DOI] [PubMed] [Google Scholar]
- 130.Dummer R (2008) Phase i trial of oral vorinostat in combination with bexarotene in advanced cutaneous T-cell lymphoma. Hematologica 93
- 131.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Viviani S (2008) A phase Ii study of the hostone deacetyalse inhibitor ITF2357 in relapsed/ refractory Hodgkin’s lymphoma patients. J Clin Oncol 26(suppl): abstract 8532
- 133.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]