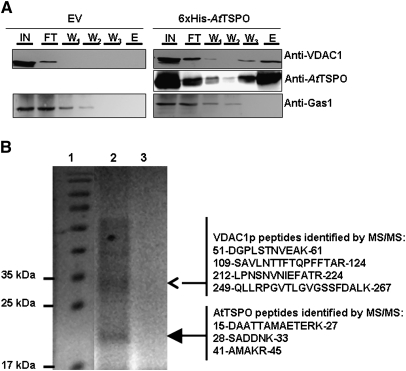
Fig. 6.
Yeast VDAC1p copurifies with 6×His-AtTSPO. The expressed 6×His-AtTSPO in yeast was solubilized from total microsomes and affinity purified using a Ni-NTA matrice, with microsomes from the yeast strain transformed with the empty vector (EV) used as control. (A) The input (IN), flowthrough (FT), the three successive washes (respectively W1, W2, and W3) and the elution fraction (E) were analysed by immunoblotting for the presence of AtTSPO, with VDAC1p and Gas1p as control membrane proteins. (B) The elution from 6×His-AtTSPO strain (lane 2) or the empty vector strain (lane 3) were separated by SDS-PAGE alongside Mr markers (lane 1); after blue colloidal staining of the polypeptides the main bands in lane 2 (arrowheads) were excised and in-gel tryptic-digested and the resulting peptides analysed by MALDI TOF/TOF; the identified peptide for each polypeptide are shown on the right with their start and end in the primary sequence of the MS/MS identified protein.