Abstract
In diatoms, metabolic activity during long dark periods leads to a chlororespiratory electron flow, which is accompanied by the build-up of a proton gradient strong enough to activate the diadinoxanthin (Ddx) de-epoxidation reaction of the Ddx cycle. In the present study, the impact of chlororespiration on non-photochemical quenching (NPQ) of chlorophyll fluorescence and the regulation of the Ddx cycle in the diatom Thalassiosira pseudonana was investigated by manipulation of the redox state of the photosynthetic electron transport chain during darkness. The response of a transfer of T. pseudonana cells from growth light conditions to 60 min darkness was found to depend on oxygen: in its presence there was no significant reduction of the PQ pool and no de-epoxidation of Ddx to diatoxanthin (Dtx). Under anaerobic conditions a high reduction state of the electron transport chain and a slow but steady de-epoxidation of Ddx was observed, which resulted in a significant accumulation of Dtx after 60 min of anaerobiosis. Unexpectedly, this high concentration of Dtx did not induce a correspondingly high NPQ as it would have been observed with Dtx formed under high light conditions. However, the sensitivity of NPQ to Dtx in cells kept under dark anaerobic conditions increased during reoxygenation and far-red (FR) light illumination. The results are discussed with respect to the activation of the de-epoxidation reaction and the formation of NPQ and their dependence on the extent of the proton gradient across the thylakoid membrane.
Keywords: Anaerobiosis, chlororespiration, diadinoxanthin cycle, diatom, NPQ
Introduction
In diatoms, the transition of the photosynthetic apparatus from a light harvesting to a photoprotective state is characterized by the non-photochemical quenching (NPQ) of chlorophyll (Chl) a fluorescence, and the cycling of electrons around photosystem II (PSII) and/or photosystem I (PSI) (Lavaud et al., 2002a, b, 2004; Wilhelm et al., 2006). Although the mechanistic basis of NPQ in diatoms shares common features with the NPQ mechanism of vascular plants (Miloslavina et al., 2009), i.e. the build-up of a transmembrane proton gradient and the formation of de-epoxidized xanthophyll cycle pigments, significant differences exist (Ruban et al., 2004; Lavaud and Kroth, 2006; Grouneva et al., 2008a, b). The xanthophyll cycle of diatoms comprises two pigments, diadinoxanthin (Ddx) and diatoxanthin (Dtx), and a strict correlation between NPQ and the concentration of Dtx exists under different illumination and growth conditions (Lavaud et al., 2002c). Recently, it has been shown that in Phaeodactylum tricornutum acidification of the lumen is needed for the development of NPQ (Lavaud and Kroth, 2006), switching the xanthophylls to an ‘activated’ state, probably via the protonation of light-harvesting antenna proteins, as in vascular plants (Ruban et al., 2004). However, once the NPQ has developed, it seems to be independent of the presence of the proton gradient (Goss et al., 2006).
In illuminated diatom cells, the major component of NPQ is the energy-dependent quenching (qE), which almost solely relies on the Dtx-dependent quenching (Lavaud et al., 2002b). So far, no evidence for a state transition-dependent quenching (qT) or a reaction centre-based quenching mechanism was found in diatoms (Owens, 1986; Ting and Owens, 1994). In addition, diatoms are known actively to transport and accumulate inorganic carbon within their cells (Beardall, 1989), which strongly reduces the photoinhibitory quenching of Chl fluorescence (qI) under excess irradiance (Ting and Owens, 1994).
An important difference to vascular plants concerns the Ddx de-epoxidase (DDE), the enzyme catalysing the reaction from Ddx to Dtx. Unlike the violaxanthin de-epoxidase of vascular plants, which needs pH values of less than pH 6 for significant activity, the DDE can be activated at almost neutral pH values (Jakob et al., 2001). It was, therefore, suggested that the de-epoxidation of Ddx in P. tricornutum during prolonged dark periods (Jakob et al., 1999) can be explained by the generation of a weak ΔpH which is, however, sufficient to activate the DDE. This conclusion was derived from the fact that a dark incubation in the presence of an uncoupler prevented both the formation of Dtx and NPQ. The generation of this proton gradient was attributed to an active chlororespiratory electron flow.
The original concept of chlororespiration in Chlamydomonas reinhardtii comprises a thylakoid electron transport pathway involving an NAD(P)H plastoquinone oxidoreductase and a plastoquinol oxidase activity (Bennoun, 1982). The concept also included the putative generation of a proton gradient by the electron transport through the PQ pool. This original scheme of chlororespiration had to be modified due to the emergence of new biochemical and genetic data, in particular from vascular plants, regarding the nature of the PQ reductase and oxidase. In higher plant chloroplasts there is evidence that a Ndh complex fulfils the role of a NAD(P)H-PQ reductase (Burrows et al., 1998; Field et al., 1998; Sazanov et al., 1998). Furthermore, this membrane-bound Ndh complex shows genome sequence similarities to the proton-translocating mitochondrial type I NADH dehydrogenase (Shinozaki et al., 1986). With regard to the PQ oxidizing side of the chlororespiratory pathway, a chloroplast-targeted quinol oxidase was characterized in immutans mutants of Arabidopsis thaliana (Carol et al., 1999). This putative plastid terminal oxidase (PTOX) shows homologies to the mitochondrial alternative oxidase (AOX) (Wu et al., 1999) and probably uses molecular oxygen to oxidize the PQ pool (Cournac et al., 2000). Furthermore, it was shown that this PTOX is orientated towards the stromal phase of the thylakoid membrane (Lennon et al., 2003) and is probably non-electrogenic (Cournac et al., 2000). Therefore, PTOX is not involved in a proton translocation across the thylakoid membrane. Instead, the generation of a chlororespiratory proton gradient could be explained by the existence of an electrogenic Ndh1 complex (Nixon et al., 2000) or by other mechanisms (Rappaport et al., 1999).
A characteristic of chlororespiration is the reduction of the PQ pool in darkness by stromal reducing equivalents. The identity of the electron donors remains to be clarified and could be dependent on the plant species (Nixon, 2000). Furthermore, the transfer of reducing equivalents and adenylates from the cytosol and/or mitochondria to the chloroplast may be crucial in maintaining chlororespiratory activity during darkness (Bennoun, 1994; Hoefnagel et al., 1998). In vascular plants a sustained reduction of the PQ pool during darkness was observed during anaerobic incubation (Harris and Heber, 1993; Haldimann and Strasser, 1999; Haldimann and Tsimilli-Michael, 2002, 2005). Under these conditions the respiratory activities of both the mitochondrion and the chloroplast are inhibited. The electron pressure on the plastoquinone pool will increase due to the accumulation of NAD(P)H and due to a prevention of the PQ pool oxidation by molecular oxygen or by a plastid terminal oxidase (Yoshida et al., 2006).
In algae, chlororespiration is often coupled to a non-photochemical quenching of Chl fluorescence (Büchel and Wilhelm, 1990; Wilhelm and Duval, 1990; Ting and Owens, 1993; Jakob et al., 1999). Therefore, in these organisms the generation of a proton gradient during the course of chlororespiration was postulated. In the diatom P. tricornutum, this proton gradient was strong enough to activate the Ddx de-epoxidation reaction of the Ddx cycle during long dark periods (Jakob et al., 1999). Further support for a chlororespiratory induced activation of the XC cycle in P. tricornutum is derived from studies of cells pretreated with DCMU where a significant amount of Dtx was accumulated during high-light illumination (Eisenstadt et al., 2008; Grouneva et al., 2009). This implies that in P. tricornutum stromal reductants were involved in a chlororespiratory electron transport, which contributed to the acidification of the thylakoid lumen. On the other hand, in Cyclotella meneghiniana cells no accumulation of Dtx was observed during high-light illumination in the presence of DCMU (Grouneva et al., 2009). Thus, in diatoms a large metabolic heterogeneity could be present (see Wilhelm et al., 2006) which is also indicated by the pronounced difference in the genome structure between the pennate diatom P. tricornutum (Armbrust et al., 2004) and the centric diatom T. pseudonana (Bowler et al., 2008).
In the present work, T. pseudonana was used to examine the reduction state of the PQ pool, the oxidation kinetics of P700, the extent of NPQ, and the activity of the Ddx cycle under chlororespiratory conditions. Anaerobic conditions were used to manipulate the redox state of the photosynthetic electron transport chain during darkness in order to gain further insight into the impact of chlororespiration on NPQ and the regulation of the Ddx cycle. In addition, a subsequent period of reoxygenation or FR-light illumination was used to relieve the cells from the anaerobic stress. Reoxygenation and FR-light illumination was further presumed to increase the proton gradient across the thylakoid membrane and to allow the investigation of the modulation of the Dtx-dependent NPQ by the extent of the ΔpH.
Materials and methods
Algal cultures, preparation of samples, and experiments protocol
T. pseudonana (CCMP 1335, USA) cells were grown as air-lift cultures with silica-enriched f/2 medium (Guillard and Lorenzen, 1972) at 20 °C in a 14/10 h light/dark cycle (80 μmol photons m−2 s−1). Unless otherwise stated, cells were harvested by centrifugation (10 min at 3000 g and 18 °C) and concentrated to a Chl a content of 100–150 μg ml−1. The high cell density was chosen to achieve anaerobic conditions in a relatively short period of time.
Oxygen measurements, slow fluorescence kinetics
Simultaneous measurements of oxygen evolution/consumption and chlorophyll fluorescence (Fig. 1) were performed at 20 °C with a Clark type oxygen electrode (MI730, Microelectrodes Inc., Bedford, NH, USA) and a PAM 101 fluorometer (Walz GmbH, Germany). Slow changes of variable chlorophyll fluorescence were recorded using the saturating pulse method according to Schreiber et al. (1994). Saturating light pulses (800 ms; 3500 μmol photons m−2 s−1) were applied in the experiments using a PAM 101 fluorometer. Chlorophyll a fluorescence measurements using the Dual-PAM-100 (Walz GmbH, Germany) were performed at room temperature. Saturating pulses had a duration of 800 ms and a light intensity of 12 000 μmol photons m−2 s−1. This high light intensity was necessary due to the high cell concentration in the samples (see above). Non-photochemical quenching (NPQ) was calculated as (Schreiber et al., 1994). Fm denominates the maximal fluorescence value in dark-adapted cells and is the maximal fluorescence value in illuminated cells. However, in the dark experiments involving aerobic/anaerobic transitions, Fm represents the maximum fluorescence value induced by a saturating light pulse obtained at a certain time point of the total measuring period. Fm was usually achieved at the beginning of the anaerobic period, but could also occur at a later point of the incubation period. was assigned to the maximum fluorescence value resulting from all other saturating light pulses given during the course of the dark-aerobic/anaerobic periods. Irrespective of the experimental conditions, the term ‘minimal fluorescence’ was assigned to the fluorescence signal triggered by the measuring light of the Dual-PAM.
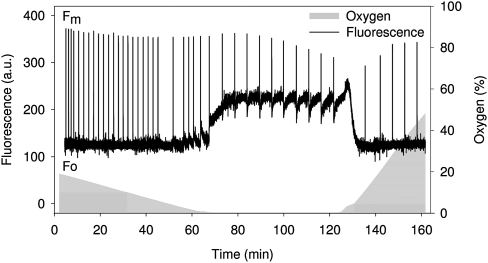
Fig. 1.
Slow fluorescence kinetics of intact cells of T. pseudonana during dark incubation. Cells (Chl a content of 2 μg ml−1) were harvested from the growth light conditions and used immediately. The oxygen concentration of the medium is depicted by the shaded area. Before the start of measurements, samples were bubbled with nitrogen to reduce the initial oxygen concentration to approximately 20% of oxygen-saturated water. Oxygen was completely removed from the medium after 60–70 min and reintroduced after 125 min of dark incubation.
Fast fluorescence induction kinetics/830 nm absorption changes measurements
Fast fluorescence induction kinetics (time scale of ms) and absorption changes at 830 nm were simultaneously measured during the saturating pulse applied with the Dual-PAM-100 (Chlorophyll a fluorescence and P700 photosynthesis analyser equipped with a P700-dual-wavelength-emitter at 830 nm and 875 nm; Walz GmbH, Germany). Stirring of the samples was avoided during data recording in order to minimize noise in the P700 absorbance signal. P700 oxidation/reduction was measured in the transmission mode and calculated as the signal at 875 nm minus the signal at 830 nm. The signal difference (displayed as the photocurrent measured with the Dual-PAM) was proportional to the absorption change at 830 nm. Thus, oxidation of P700 became visible as a signal increase (P700+ relative), whereas re-reduction of P700 led to a signal decrease.
It is known that plastocycanin (PC) contributes significantly to the absorbance changes at 830 nm (Klughammer and Schreiber, 1991; Schansker et al., 2003). In diatoms, PC is replaced by a cytochrome c (Cyt c) as the electron donor of PSI (Sandmann et al., 1983). However, Cyt c should not contribute to the absorbance change at 830 nm.
Pigment data
To ensure the comparability of experimental conditions for the determination of NPQ and the pigment composition, the samples for pigment analysis were collected at different time points of the different treatments directly from the measuring cuvette of the Dual-PAM and were rapidly frozen in liquid nitrogen. This means that for a certain time point, NPQ and DES were obtained from the same sample, but new samples had to be prepared for the different time points. To yield comparable incubation periods under anaerobic conditions for all samples, the sudden increase of the minimal fluorescence was used as a marker for the beginning of the anaerobic phase (see results section). To prove that the increase of the minimal fluorescence is a marker for the complete absence of oxygen, a control experiment with glucose/glucose oxidase (according to McTavish et al., 1989) was performed, where a comparable increase in the minimal fluorescence was observed (data not shown).
For pigment analysis by HPLC the frozen cells were slowly defrosted and collected on a glass fibre filter, pigments were extracted with a medium consisting of 90% methanol/0.2 M ammonium acetate (90/10, v/v) and 10% ethyl acetate. The extracts were centrifuged for 2 min at 13 000 g (centrifuge 5417C, Eppendorf, Germany) and injected into the HPLC column. Pigment analysis was carried out on a HPLC system (Waters, Millipore, Eschborn) equipped with a Nucleosil ET 250/8/4, 300-5, C18 column (Macherey and Nagel, Düren, Germany). Pigments were analysed and quantified according to the methods used by Wilhelm et al. (1995) and Lohr and Wilhelm (2001). Ddx de-epoxidation and Dtx epoxidation are depicted as changes in the de-epoxidation state of the Ddx cycle pigment pool calculated as Dtx (Ddx+Dtx)−1.
Results
Effects of dark-anaerobiosis on the kinetics of fluorescence induction and oxidation of P700
Figure 1 shows the response of chlorophyll fluorescence from dark-adapted suspensions of T. pseudonana cells to changes in the oxygen concentration. During the first 60 min of dark-adaptation, while oxygen was still present in the medium, there was no significant change in the minimal fluorescence, but a slight decrease in the maximal fluorescence (). Mitochondrial respiration of the cells gradually decreased the oxygen concentration in the measuring chamber. In this way, within approximately 60 min of dark incubation, anaerobic conditions were achieved. Within the first 10 min of anaerobic conditions a significant increase in the minimal fluorescence was observed. After an initial rise, started to decrease constantly due to non-photochemical quenching processes. After approximately 60 min of anaerobic incubation, the samples were aerated to re-introduce oxygen and, immediately, the minimal fluorescence decreased. also decreased, followed by a slow increase over 20 min as NPQ relaxed. Note that the initial rise of the minimal fluorescence at the beginning of reoxygenation was not observed in measurements using the Dual-PAM (data not shown). It could be assumed that this initial rise in the minimal fluorescence represents an experimental artefact. Nevertheless, the simultaneous measurement of chlorophyll fluorescence and oxygen concentration was important to prove the correlation of the changes in the fluorescence signal with the induction of/release from complete anaerobic conditions.
It has to be emphasized that a FR-light illumination under anaerobic conditions induced the same rapid decrease of the minimal fluorescence and as observed under reoxygenation. However, the slow relaxation of NPQ described for reoxygenation was not observed under FR-light illumination (data not shown).
Measurements of fast fluorescence induction kinetics provided information about the reduction state of the PQ pool. Upon excitation of a dark-adapted photosynthetic sample, the first signal level detected in a fluorescence transient is labelled as O, while its intensity is usually denoted as the minimal fluorescence Fo. At the O-level, QA is considered to be maximally (but not totally) oxidized. Continuous exciting illumination then drives Chl a fluorescence through two inflections: J (after approximately 2 ms) and I (after approximately 30–50 ms). Finally, a peak (P) is reached at approximately 500 ms complementing the so-called OJIP transients (Lazár, 2006; Papageorgiou et al., 2007). The J-level of the fast fluorescence induction curve is supposed to reflect light-driven accumulation of reduced QA (Strasser et al., 1995). Since QA is in redox equilibrium with the PQ pool, the J-level also represents an estimate of changes in the reduction state of the PQ pool (Tóth et al., 2007). An increase in the J-level of transient fluorescence is then correlated with a more reduced PQ pool. After dark incubation, this could be due to electron donation from electron sources located in the chloroplast stroma (Haldimann and Strasser, 1999; Schansker et al., 2005).
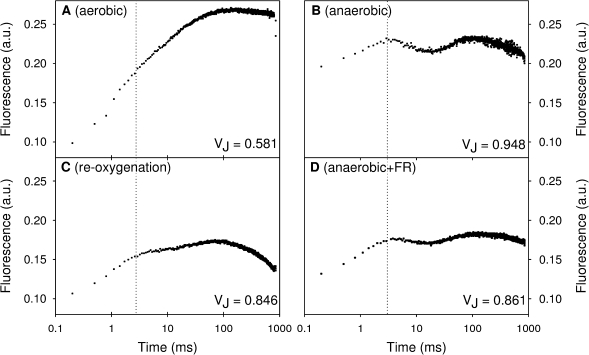
Figure 2 shows the OJIP-transients of T. pseudonana cells under four different conditions. Figure 2A presents an example of the Chl a fluorescence induction kinetics of cells after 60 min of dark-aerobic conditions. The shape of the fast fluorescence kinetics did not change in samples measured at different time points during the 60 min of dark-aerobic adaptation (data not shown). However, in the absence of oxygen, 60 min of dark incubation drastically increased the fluorescence intensity of the J-step compared with dark incubation under aerobic conditions (indicated by VJ, the relative variable fluorescence at the J-level; Fig. 2). The fluorescence transient showed a dip between the J and the P-level after 60 min dark incubation under anaerobiosis. This indicates that, under these conditions, a part of the QA was oxidized by PSI-activity before full reduction was restored. Furthermore, it was noted that the fluorescence transient rapidly decreased again after reaching the P-level, which was not observed in dark-adapted control samples.
Fig. 2.
Examples of fast Chl a fluorescence induction measurements of dark-adapted cell suspensions of T. pseudonana exposed to different experimental conditions: (A) dark-aerobic incubation; (B) after 60 min of dark-anaerobic incubation; (C, D) 60 min of anaerobic incubation followed by a 30 min period of reoxygenation or illumination with FR light (720 nm), respectively. The dashed line indicates the J-level. VJ is the relative variable fluorescence at the J-step at 2 ms calculated as: VJ=(F2ms–Fm)/(Fm–Fo).
It could be argued that the saturating pulses itself applied every 15 min during dark-anaerobic conditions influenced the redox state of the PQ-pool and thus led to a higher J-level of the fluorescence transients. However, no significant differences in the fluorescence transients after dark-anaerobic incubation were observed using the following protocol: (i) saturating light pulses were given at time points 0, 1, 2, 5, 10, 15, 30, and 60 min, (ii) no additional saturating light pulses were given between the first and the last pulse at the time points 0 min and 60 min, respectively.
After a 60 min period of anaerobic dark incubation, a 30 min period of reoxygenation or FR-illumination was applied. The re-introduction of oxygen immediately decreased the initial fluorescence (Fig. 2C) to almost the same level as observed in dark-adapted control samples (Fig. 2A), which is comparable to the decrease of the minimal fluorescence shown in Fig. 1. In addition, the lower J-level after reoxygenation (Fig. 2C) compared with anaerobic dark conditions indicated a partial oxidation of the PQ pool. FR-illumination was slightly less effective in decreasing the O-level and the J-level compared with reoxygenation (Fig. 2D).
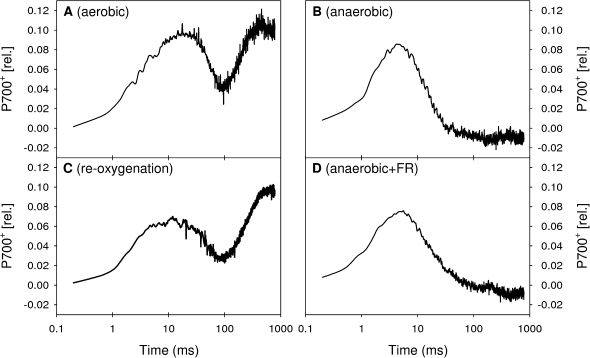
To gain further insight into the capacity of the electron flow from the PQ pool to PSI and the electron transfer steps following PSI, measurements of P700 absorbance changes upon illumination by a saturating light pulse were performed simultaneously with the measurements of fast fluorescence induction curves (Fig. 3). The light-induced P700 absorbance changes provide information about the kinetics and extent of P700 oxidation. In the measurements as performed in the present study an upward signal corresponds to the oxidation of P700, whereas a downward signal is due to the reduction of P700+. In control cells under dark-aerobic conditions (Fig. 3A) three different phases could be distinguished in the P700 absorbance changes: (i) the initial oxidation of P700 during the first 20–30 ms of the saturating illumination, (ii) a transient reduction of P700 between the time point of 30–150 ms, and (iii) the final oxidation of P700 after 300–400 ms (Grouneva et al., 2009). The transient reduction of P700 is due to an inactive FNR on the PSI acceptor side, which inhibits the electron transfer to NADP+ (Maxwell and Biggins, 1977; Schansker et al., 2005; Tóth et al., 2007). Thus, the transient reduction of P700 reflects electrons originating from PSII or an electron inflow into the PQ pool from stromal sources. Once the FNR is activated (after approximately 150 ms of the saturating illumination) the acceptor side of PSI has sufficient capacity to maintain P700 in the oxidized state.
Fig. 3.
Examples of measurements of P700 absorbance changes at 830 nm in cell suspensions of T. pseudonana exposed to different experimental conditions: (A) dark-aerobic incubation; (B) 60 min of dark-anaerobic incubation; (C, D) 60 min of anaerobic incubation followed by a 30 min period of reoxygenation or illumination with FR light (720 nm), respectively.
After dark incubation in the absence of oxygen, a significantly different P700 absorbance change kinetics was observed (Fig. 3B). The initial oxidation of P700 was followed by a rapid and complete re-reduction, whereas a final phase of oxidation of P700 was absent. The addition of DCMU to dark-incubated anaerobic samples did not alter the P700 absorbance change kinetics (data not shown), which demonstrates that the majority of electrons reducing P700 under anaerobic conditions did not originate from PSII. Instead, these electrons must have been supplied by a stromal source feeding electrons into the PQ-pool under dark-anaerobic conditions. This suggestion is supported by the increased reduction state of the PQ-pool detected by the simultaneous measurements of the fast fluorescence kinetics (see above).
A 30 min period of reoxygenation of dark-incubated anaerobic samples (Fig. 3C) regenerated the shape of the P700 absorbance change kinetics as observed in control cells under dark-aerobic conditions (Fig. 3A) with a transient reduction of P700+ followed by a final oxidation of P700. It is noteworthy, that this recovery of P700 oxidation was already observed in measurements after 2 min of reoxygenation (data not shown). By contrast, 30 min FR-illumination of cells of T. pseudonana after a 60 min period of dark-anaerobic conditions could not re-establish the final oxidation of P700 (Fig. 3D).
Correlation of DES and NPQ during dark anaerobiosis and recovery from anaerobiosis
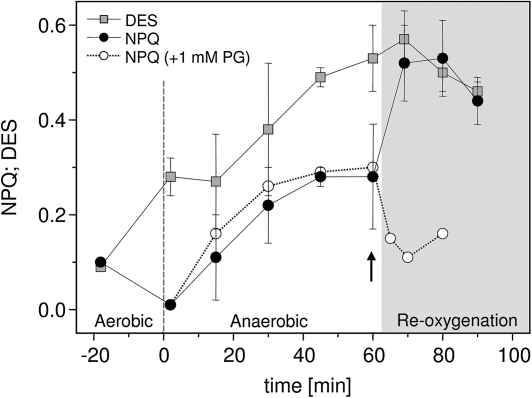
In diatoms, the light-induced increase in NPQ is strongly correlated with the Dtx concentration and the de-epoxidation state (DES) of the Ddx cycle pigment pool. Figure 4 shows NPQ and the respective DES from different time points during dark-anaerobic conditions, during the relaxation period where oxygen was re-introduced by aeration of the samples, and from the control samples under dark-aerobic conditions before the start of anaerobic conditions. In control samples, a DES of 0.09 and an NPQ of 0.1 were observed; these values did not change when samples were bubbled with air in darkness during a period of 60 min. Measurements of samples taken in the first 2 min of the anaerobic period showed that the DES increased from 0.09 to a value of about 0.28, whereas the NPQ value remained low during the first minutes of the anaerobic period. Within 60 min of dark incubation under anaerobic conditions a biphasic increase of DES and NPQ was observed. During the first 45 min there was a relatively fast concomitant increase of DES and NPQ which was followed by a much slower kinetics during the remaining dark anaerobic phase. Finally, at the end of anaerobic incubation DES and NPQ reached values of 0.53 and 0.28, respectively. It is worth mentioning that the Ddx cycle pigment pool remained constant at 225±12 mM (M Chl a)−1 during the course of the experiments shown in Fig. 4. Thus, the observed increase in the DES was caused by the enzymatic de-epoxidation of Ddx to Dtx and not by a de novo synthesis of Dtx.
Fig. 4.
Time-dependent changes of NPQ and the de-epoxidation state (DES) of the Ddx cycle pigment pool [Dtx (Ddx+Dtx)−1] in cell suspensions of T. pseudonana during prolonged dark incubation. The negative time period represents the time span required for oxygen depletion from the medium. NPQ and DES determined during that period represent the values obtained during dark-aerobic incubation. These values did not change between the onset of experiments and during 60 min of darkness when aerobic conditions were maintained. The grey area represents the reoxygenation period. Data presented are the average of 3–20 replicates with the respective standard errors. To suppress chlororespiratory activity the inhibitor of the terminal oxidase (PTOX) Propylgallate (PG, 1 mM) was added to cell suspensions directly at the start of the reoxygenation period (indicated by the arrow). A representative example of the time-course of NPQ before and after the addition of PG is shown. Three independent measurements were carried out which confirmed the results depicted in the figure.
Within the first 10 min of relaxation from anaerobic conditions by reoxygenation an immediate increase of NPQ was observed, whereas the DES still increased with the same slow kinetics as during the late anaerobic phase (see the grey area in Fig. 4). Relaxation periods of more than 10 min resulted in a concomitant decrease of NPQ and the DES. The first 10 min of illumination with FR-light also induced a further increase of NPQ and DES, as observed during reoxygenation. However, in contrast to the recovery by the introduction of oxygen, the DES and NPQ remained high during FR-light illumination periods exceeding 10 min (data not shown). An interesting result was obtained in experiments in the presence of Propylgallate (PG) which was used as an inhibitor of the PTOX. The addition of 1 mM PG directly at the beginning of the reoxygenation period resulted in a fast decrease of NPQ values in contrast to the significant increase of NPQ observed in the control samples (Fig. 4).
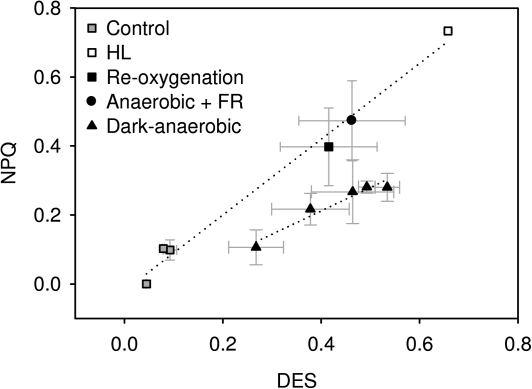
The correlation between the DES of the Ddx cycle pigment pool and NPQ indicates the sensitivity of NPQ to Dtx. Thus, from the time-course of changes in DES and NPQ as shown in Fig. 4 it is evident that the sensitivity of NPQ to Dtx formed under anaerobic conditions must have drastically increased during the transition to dark-aerobic conditions. This conclusion was corroborated by the results depicted in Fig. 5, which compares the sensitivity of NPQ to Dtx (indicated by the correlation of the two shown as dotted lines in the figure) formed under a variety of aerobic and anaerobic conditions. NPQ was less sensitive to Dtx formed during darkness in the absence of oxygen compared with all other experimental conditions. The re-introduction of oxygen or FR illumination strongly increased the sensitivity of NPQ to Dtx and the same correlation between NPQ and Dtx was observed as during HL-illumination.
Fig. 5.
Correlation between NPQ and de-epoxidation state (DES) of the Ddx cycle pigment pool of cell suspensions of T. pseudonana exposed to different conditions. NPQ and the DES were determined in the dark at the onset of experiments and after an illumination period of 10 min with low light (LL, 20 μmol photons m−2 s−1) intensities (grey squares); at different time points during 60 min of dark-anaerobic conditions (closed triangles); after a 30 min reoxygenation period following the 60 min of dark-anaerobic conditions (closed square); after a 30 min relaxation period in the presence of far-red (FR, 720 nm) light illumination following the 60 min of dark-anaerobic conditions (closed circle); after a 5 min period of high light (HL, 825 μmol photons m−2 s−1) treatment (open squares). Cells were harvested by centrifugation and concentrated to a Chl a content of ∼30 μg ml−1 for the LL and HL experiments, and to a Chl a content of 100–150 μg ml−1 for the remaining experiments. The difference in Chl a content did not affect NPQ and DES determined at the onset of experiments. Data presented are the average of 3–20 replicates with the respective standard errors.
Effects of nigericin on cells under dark-anaerobic conditions
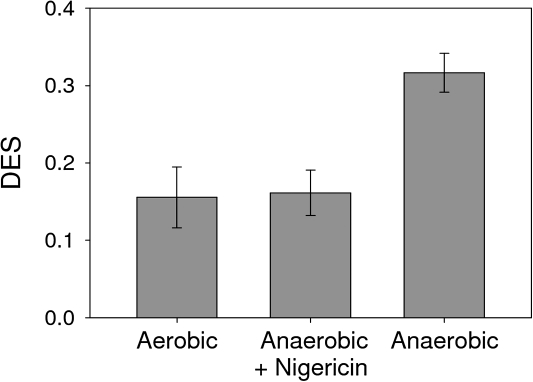
The increase of the DES following a transition to anaerobic conditions indicated that a small proton gradient was already present at the beginning of dark-anaerobic conditions. To confirm this assumption, the uncoupler nigericin was applied at the beginning of a dark-anaerobic period (Fig. 6). In control samples kept in dark-aerobic conditions a DES of 0.15 was observed which was due to Dtx already present in the cells at the beginning of dark incubation. As observed before, dark-anaerobic conditions led to a significant increase of DES to a value of 0.32. The addition of the uncoupler nigericin is expected to prevent the generation of a proton gradient across the thylakoid membrane, thereby inhibiting the activation of the Ddx de-epoxidase. Indeed, no increase in the DES was observed during a dark incubation under anaerobiosis in the presence of nigericin.
Fig. 6.
Effect of nigericin on the de-epoxidation state (DES) of the Ddx cycle pigment pool in samples of T. pseudonana exposed to different experimental conditions: dark-aerobic conditions and 30 min of dark-anaerobic conditions in the presence or absence of nigericin. Cells were harvested by centrifugation and concentrated to a Chl a content of ∼30 μg ml−1. Final concentration of nigericin was 40 mM. Data presented are the average of three replicates with the respective standard deviations.
Discussion
In the present study, dark incubation under anaerobic conditions was used to increase chlororespiratory electron flow in cells of T. pseudonana. Anaerobiosis blocks mitochondrial respiration and increases the fermentative degradation of carbohydrates (Pasteur effect). Under these conditions reduction equivalents will accumulate in the cell (Rebeille and Gans, 1988). Via the malate/oxalacetate shuttle the reductants can be imported into the chloroplast stroma and may lead to a non-photochemical reduction of the PQ pool (Hoefnagel et al., 1998). In addition, anaerobiosis should prevent an oxidation of the PQ pool by molecular oxygen (non-enzymatically) or by a PTOX. The measurements of PAM fluorescence, fast fluorescence induction kinetics, and P700 absorbance changes in the present study provide evidence for a high degree of reduction in the chloroplast stroma and a strong electron pressure into the PQ pool of T. pseudonana.
The transfer of T. pseudonana cells from growth light to dark-anaerobic conditions resulted in an accumulation of and a significant reduction of the PQ pool, which could be deduced from the marked increase in the minimal fluorescence and the drastic increase of the J-level, respectively. These results are comparable to measurements under anaerobiosis in vascular plants (Harris and Heber, 1993; Tóth et al., 2007). The measurements of the P700 absorbance kinetics revealed that the anaerobic conditions inhibited the final oxidation of P700 during a saturating light pulse. In control samples, the activation of the FNR facilitates the oxidation of P700 after approximately 150 ms of saturating illumination, which is comparable to the fast PSI reoxidation in cells of Trebouxia (Ilík et al., 2006). The persisting reduction of P700 under anaerobiosis is consistent with an inactivation of the PSI acceptor side due to the accumulation of reduced pyridine nucleotides. In addition, the inhibition of the Mehler reaction (see Asada et al., 1992, and references therein) could contribute to the sustained reduction of P700. Recently, Kuvykin et al. (2008) have shown that the electron flux diverted from PSI to oxygen can be as large as 40% of the total electron flow passing through PSI. This assumption is supported by a study on algae (Wagner et al., 2006) where it was shown that, under dynamic light conditions, up to 35% of photosynthetic electrons can be consumed in alternative electron sinks, which depend on the reduction of oxygen. In the light of these results, it could be assumed that molecular oxygen did not function as an alternative electron acceptor at PSI (Asada, 1999; Badger et al., 2000; Papageorgiou and Govindjee, 2005) under the anaerobic conditions of the present study.
In vascular plants, the anaerobic increase in the minimal fluorescence and in the J-level of fast fluorescence induction kinetics relaxed rapidly after either reoxygenation or illumination with FR-light (Harris and Heber, 1993; Haldimann and Strasser, 1999). These results are highly comparable to the fluorescence measurements in cells of T. pseudonana during recovery from anaerobiosis. In addition it is shown that after re-introduction of oxygen, the oxidation of P700 during a saturating light flash was immediately restored in the T. pseudonana cells. In principle, the relaxation by reoxygenation could be explained by a re-activation of mitochondrial activity and a very fast oxidation of stromal reductants. Thereby, the strong electron pressure on the PQ pool will be released and oxidized electron acceptors will be available at PSI. However, Haldimann and Strasser (1999) argued that the pool of reductants, which had accumulated during anaerobiosis, could not be depleted by a short-term relaxation. Instead, molecular oxygen could be an obligatory component for the oxidation of the PQ pool (Harris and Heber, 1993), but also act as an alternative electron acceptor at PSI (see above). In addition, the possibility that activation of a plastidal oxidase is likely in the presence of oxygen and, thus, could contribute to an oxidation of the PQ pool, has to be considered. In particular, the presence of such a chlororespiratory electron flow is important in the light of the increase in the NPQ-sensitivity of Dtx during reoxygenation (see below).
By contrast to reoxygenation, FR-light illumination of anaerobic cells of T. pseudonana could not restore P700 oxidation during a saturating light pulse. This result is in line with the activation of a cyclic electron transport around PSI where electrons are transferred back to P700 and kept in a reduced state (Joliot and Joliot, 2006). Two different pathways have been described for the cycling of electrons around PSI, both of them including an electron transport via the PQ pool (Joliot and Joliot, 2006; Shikanai, 2007). Therefore, it remains an open question why the PQ pool is becoming oxidized in our present experiments while the PSI electron cycle at the same time should reduce it again.
A 60 min period under dark-anaerobic conditions induced a strong accumulation of Dtx in T. pseudonana. In diatoms, two prerequisites must be fulfilled to achieve an efficient de-epoxidation of Ddx to Dtx. First, the DDE has to be activated by an acidification of the thylakoid lumen. Compared with the very fast de-epoxidation of Ddx under high-light illumination (Lavaud et al., 2002c), the slow kinetics of Dtx accumulation during dark-anaerobic conditions indicates the presence of a rather weak proton gradient, which was unable to activate the DDE fully. However, Jakob et al. (2001) have shown that though the isolated DDE has a pH optimum of 5.5, enzyme activity can already be observed at neutral pH values of 7.2. Consequently, even a weak proton gradient would suffice to induce a slow conversion of Ddx to Dtx. The presence of a ΔpH was confirmed by experiments in the presence of the uncoupler nigericin. The addition of nigericin at the beginning of dark-anaerobic incubation inhibited the accumulation of Dtx. It is a matter of debate how the proton gradient was generated. With respect to the high energy- and redox-charge of the cell during anaerobiosis, the fermentative degradation of carbohydrates could provide ATP (Gfeller and Gibbs, 1985; Finazzi et al., 1999), which can be imported into the chloroplast via the triose-phosphate shuttle (Hoefnagel et al., 1998) or via the recently identified nucleotide transporter in diatoms (Ast et al., 2009). Finally, a reverse activity of the ATPase could have sustained a proton gradient across the thylakoid membrane (Gilmore and Björkman, 1995; Joliot and Joliot, 1980).
The second prerequisite for an efficient accumulation of Dtx is the suppression of the fast epoxidation of Dtx by the DEP, which would otherwise rapidly convert Dtx back into Ddx. During high light-illumination DEP is inactivated by the build-up of a strong proton gradient (Goss et al., 2006), which was most probably not the case during the anaerobic conditions of the present study (see above). However, like the zeaxanthin epoxidase of higher plants (Büch et al., 1995), Dtx epoxidase requires O2, FAD, and NAD(P)H to re-introduce the epoxy group into the Dtx molecule. Therefore, it is reasonable to assume that the absence of oxygen suppressed the Dtx epoxidase activity and thus, facilitated the Ddx de-epoxidation during anaerobiosis.
An important result of the present study was the reduced sensitivity of NPQ to Dtx formed during anaerobic conditions in darkness compared with HL-induced Dtx. This is different from results obtained in P. tricornutum, where Dtx formed during prolonged dark incubation had the same efficiency in the induction of NPQ as Dtx induced by a light-driven proton gradient (Jakob et al., 1999). As in higher plants (Horton et al., 1996), the acidification of the thylakoid lumen in diatoms seems to be essential for the development of NPQ (Lavaud and Kroth, 2006). Again, as in higher plants, it was suggested that this activation of NPQ involves the protonation of PSII antenna proteins leading to a conformational change of the antenna system. We suggest that, in our present experiments, the weak ΔpH was, on the one hand, able to activate the DDE but that, on the other hand, significant protonation of LHC antenna sites did not occur. This resulted in a low NPQ despite a strong accumulation of Dtx. Immediately upon the re-introduction of oxygen or illumination with FR-light, NPQ increased significantly in samples previously adapted to dark-anaerobic conditions, while the Dtx concentration remained virtually unchanged. Hence, the sensitivity of NPQ to Dtx during the reoxygenation period or the FR-light illumination was the same as in cells illuminated by saturating light intensities. This can be explained if both reoxygenation and FR-illumination caused an increase in the ΔpH across the thylakoid membrane. As suggested above, reoxygenation should induce the oxidation of the PQ pool by the plastidal oxidase and thereby promote a chlororespiratory electron flow from the stromal pool of accumulated reducing equivalents via the PQ pool to oxygen. Hence, with the prerequisite of a proton-translocating type-1 Ndh this chlororespiratory electron flow could have contributed to a proton gradient across the thylakoid membrane (Nixon, 2000, Grouneva et al., 2009) upon the reintroduction of oxygen. The assumption of an electrogenic chlororespiratory electron flow in T. pseudonana under the conditions of the present study is supported by experiments where PG was added to anaerobic cell suspensions directly at the beginning of the reoxygenation period. PG is known to inhibit the activity of PTOX in algal cells (Cournac et al., 2000; Bennoun, 2001). The fast decrease of NPQ in the presence of PG suggests that the inhibition of the PTOX resulted in the dissipation of an existing (weak) proton gradient and in addition prevented the further increase of the ΔpH as observed in control samples. Further indication of an increase in the proton gradient is derived from the fact that the epoxidation of Dtx by the DEP was inhibited for the first 10 min of the reoxygenation period. In the presence of oxygen this can be explained only by an inhibition of the DEP by a strong proton gradient across the thylakoid membrane (Goss et al., 2006). For reoxygenation periods longer than 10 min it could be assumed that mitochondrial activity slowly deprived the pool of accumulated reducing equivalents. Following the argument above, this would also diminish the chlororespiratory electron flow and the associated ΔpH. As a consequence, the inhibition of the DEP will be abolished, which results in the epoxidation of Dtx back to Ddx.
FR-light illumination is assumed to drive cyclic electron transport around PSI, which is known to increase the proton gradient across the thylakoid membrane (Arnon, 1984). Apparently, this proton gradient was strong enough to increase the sensitivity of NPQ to Dtx. In contrast to reoxygenation, no epoxidation of Dtx by the DEP was observed during a 30 min period of FR-light illumination. In this case, the DEP was most probably kept inactive by both the absence of oxygen and the proton gradient.
In conclusion, the data of the present study show that, in diatoms, the chlororespiratory pathway can be an important regulatory tool to control energy dissipation during the transition from dark to full sunlight.
Acknowledgments
We thank Irina Grouneva for discussion and critical comments on the manuscript. This work was supported by project SFRH/BD/23505/2005, funded by Fundação para a Ciência e a Tecnologia, Portugal.
Glossary
Abbreviations
- AOX
alternative oxidase
- Chl
chlorophyll
- DDE
diadinoxanthin de-epoxidase
- Ddx
diadinoxanthin
- DES
de-epoxidation state of the Ddx cycle pigment pool
- Dtx
diatoxanthin
- Fd
ferredoxin
- FNR
ferredoxin-NADP+-oxidoreductase
- FR
far-red
- NPQ
non-photochemical quenching of Chl a fluorescence
- P700
reaction centre pigments of PSI
- PQ
plastoquinone
- PSII
photosystem II
- PSI
photosystem I
- PTOX
plastid terminal oxidase
- QA
quinone A
References
- Armbrust EV, Berges JA, Bowler C, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- Arnon DI. The discovery of photosynthetic phosphorylation. Trends in Biochemical Sciences. 1984;9:258–262. doi: 10.1016/0968-0004(88)90016-3. [DOI] [PubMed] [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Asada K, Heber U, Schreiber U. Pool size of electrons that can be donated to P700+ as determined in intact leaves: donation to P700+ from stromal components via the intersystem chain. Plant and Cell Physiology. 1992;33:927–932. [Google Scholar]
- Ast M, Gruber A, Schmitz-Esser S, Neuhaus HE, Kroth PG, Horn M, Haferkamp I. Diatom plastids depend on nucleotide import from the cytosol. Proceedings of the National Academy of Sciences, USA. 2009;106:3621–3626. doi: 10.1073/pnas.0808862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Caemmerer SV, Ruuska S, Nakano H. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:1433–1446. doi: 10.1098/rstb.2000.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardall J. Photosynthesis and photorespiration in marine phytoplankton. Aquatic Botany. 1989;34:105–130. [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proceedings of the National Academy of Sciences, USA. 1982;79:4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochimica et Biophysica Acta, Bioenergetics. 1994;1186:59–66. [Google Scholar]
- Bennoun P. Chlororespiration and the process of carotenoid biosynthesis. Biochimica et Biophysica Acta, Bioenergetics. 2001;1506:133–142. doi: 10.1016/s0005-2728(01)00190-6. [DOI] [PubMed] [Google Scholar]
- Bowler C, Allen AE, Badger JH, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature. 2008;456:239–244. doi: 10.1038/nature07410. [DOI] [PubMed] [Google Scholar]
- Büch K, Stransky H, Hager A. FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Letters. 1995;376:45–48. doi: 10.1016/0014-5793(95)01243-9. [DOI] [PubMed] [Google Scholar]
- Büchel C, Wilhelm C. Wavelength independent state transitions and light regulated chlororespiration as mechanisms to control the energy status in the chloroplast of. Pleurochloris meiringensis. Plant Physiology and Biochemistry. 1990;28:307–314. [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. The EMBO Journal. 1998;17:868–876. doi: 10.1093/emboj/17.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. The Plant Cell. 1999;11:57–68. doi: 10.1105/tpc.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Josse EM, Joët T, Rumeau D, Redding K, Kuntz M, Peltier G. Flexibility in photosynthetic electron transport: a newly identified chloroplast oxidase involved in chlororespiration. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:1447–1454. doi: 10.1098/rstb.2000.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt D, Ohad I, Keren N, Kaplan A. Changes in the photosynthetic reaction centre II in the diatom Phaeodactylum tricornutum result in non-photochemical fluorescence quenching. Environmental Microbiology. 2008;10:1997–2007. doi: 10.1111/j.1462-2920.2008.01616.x. [DOI] [PubMed] [Google Scholar]
- Field TS, Nedbal L, Ort DR. Non-photochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiology. 1998;116:1209–1218. doi: 10.1104/pp.116.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi G, Furia A, Barbagallo RP, Forti G. State transitions, cyclic and linear electron transport and photophosphorylation in. Chlamydomonas reinhardtii. Biochimica et Biophysica Acta, Bioenergetics. 1999;1413:117–129. doi: 10.1016/s0005-2728(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Gfeller RP, Gibbs M. Fermentative metabolism of Chlamydomonas reinhardtii. Plant Physiology. 1985;77:509–511. doi: 10.1104/pp.77.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Björkman O. Temperature-sensitive coupling and uncoupling of ATPase-mediated, non-radiative energy dissipation: similarities between chloroplasts and leaves. Planta. 1995;197:646–654. [Google Scholar]
- Goss R, Pinto AE, Wilhelm C, Richter M. The importance of a highly active and delta pH-regulated diatoxanthin epoxidase for the regulation of the PSII antenna function in diadinoxanthin cycle containing algae. Journal of Plant Physiology. 2006;163:1008–1021. doi: 10.1016/j.jplph.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Grouneva I, Jakob T, Wilhelm C, Goss R. Photosynthesis. Energy from the sun. Netherlands: Springer; 2008a. Evidence for a fast, xanthophyll cycle independent NPQ mechanism in the diatom C. meneghiniana; pp. 1014–1016. [Google Scholar]
- Grouneva I, Jakob T, Wilhelm C, Goss R. A new multicomponent NPQ mechanism in the diatom. Cyclotella meneghiniana. Plant and Cell Physiology. 2008b;49:1217–1225. doi: 10.1093/pcp/pcn097. [DOI] [PubMed] [Google Scholar]
- Grouneva I, Jakob T, Wilhelm C, Goss R. The regulation of xanthophyll cycle activity and of non-photochemical fluorescence quenching by two alternative electron flows in the diatoms Phaeodactylum tricornutum and. Cyclotella meneghiniana. Biochimica et Biophysica Acta. 2009;1787:929–938. doi: 10.1016/j.bbabio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Lorenzen CJ. Yellow-green algae with chlorophyllide c. Journal of Phycology. 1972;8:10–14. [Google Scholar]
- Haldimann P, Strasser RJ. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.) Photosynthesis Research. 1999;62:67–83. [Google Scholar]
- Haldimann P, Tsimilli-Michael M. Mercury inhibits the non-photochemical reduction of plastoquinone by exogenous NADPH and NADH: evidence from measurements of the polyphasic chlorophyll a fluorescence rise in spinach chloroplasts. Photosynthesis Research. 2002;74:37–50. doi: 10.1023/A:1020884500821. [DOI] [PubMed] [Google Scholar]
- Haldimann P, Tsimilli-Michael M. Non-photochemical quenching of chlorophyll a fluorescence by oxidised plastoquinone: new evidences based on modulation of the redox state of the endogenous plastoquinone pool in broken spinach chloroplasts. Biochimica et Biophysica Acta, Bioenergetics. 2005;1706:239–249. doi: 10.1016/j.bbabio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Harris GC, Heber U. Effects of anaerobiosis on chlorophyll fluorescence yield in spinach (Spinacia oleracea) leaf discs. Plant Physiology. 1993;101:1169–1173. doi: 10.1104/pp.101.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochimica et Biophysica Acta, Bioenergetics. 1998;1366:235–255. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Ilík P, Schansker G, Kotabová E, Váczi P, Strasser RJ, Barták M. A dip in the chlorophyll fluorescence induction at 0.2–2 s in Trebouxia-possessing lichens reflects a fast reoxidation of photosystem I. A comparison with higher plants. Biochimica et Biophysica Acta, Bioenergetics. 2006;1757:12–20. doi: 10.1016/j.bbabio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Jakob T, Goss R, Wilhelm C. Activation of diadinoxanthin de-epoxidase due to a chlororespiratory proton gradient in the dark in the diatom Phaeodactylum tricornutum. Plant Biology. 1999;1:76–82. [Google Scholar]
- Jakob T, Goss R, Wilhelm C. Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum. Journal of Plant Physiology. 2001;158:383–390. [Google Scholar]
- Joliot P, Joliot A. Dependence of delayed luminescence upon adenosine triphosphatase activity in Chlorella. Plant Physiology. 1980;65:691–696. doi: 10.1104/pp.65.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P, Joliot A. Cyclic electron flow in C3 plants. Biochimica et Biophysica Acta, Bioenergetics. 2006;1757:362–368. doi: 10.1016/j.bbabio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. Analysis of light-induced absorbance changes in the near-infrared region. I. Characterization of various components in isolated chloroplasts. Zeitschrift für Naturforschung. 1991;46:233–244. [Google Scholar]
- Kuvykin I, Vershubskii A, Ptushenko V, Tikhonov A. Oxygen as an alternative electron acceptor in the photosynthetic electron transport chain of C3 plants. Biochemistry (Moscow) 2008;73:1063–1075. doi: 10.1134/s0006297908100027. [DOI] [PubMed] [Google Scholar]
- Lavaud J, Kroth PG. In diatoms, the transthylakoid proton gradient regulates the photoprotective non-photochemical fluorescence quenching beyond its control on the xanthophyll cycle. Plant and Cell Physiology. 2006;47:1010–1016. doi: 10.1093/pcp/pcj058. [DOI] [PubMed] [Google Scholar]
- Lavaud J, Rousseau B, Etienne A. In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Letters. 2002c;523:163–166. doi: 10.1016/s0014-5793(02)02979-4. [DOI] [PubMed] [Google Scholar]
- Lavaud J, Rousseau B, Etienne A. General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophycea) Journal of Phycology. 2004;40:130–137. [Google Scholar]
- Lavaud J, Rousseau B, van Gorkom HJ, Etienne A. Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiology. 2002b;129:1398–1406. doi: 10.1104/pp.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaud J, van Gorkom HJ, Etienne A. Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynthesis Research. 2002a;74:51–59. doi: 10.1023/A:1020890625141. [DOI] [PubMed] [Google Scholar]
- Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Functional Plant Biology. 2006;33:9–30. doi: 10.1071/FP05095. [DOI] [PubMed] [Google Scholar]
- Lennon A, Prommeenate P, Nixon P. Location, expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher-plant plastids. Planta. 2003;218:254–260. doi: 10.1007/s00425-003-1111-7. [DOI] [PubMed] [Google Scholar]
- Lohr M, Wilhelm C. Xanthophyll synthesis in diatoms: quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta. 2001;212:382–391. doi: 10.1007/s004250000403. [DOI] [PubMed] [Google Scholar]
- Maxwell PC, Biggins J. The kinetic behavior of P700 during the induction of photosynthesis in algae. Biochimica et Biophysica Acta. 1977;459:442–450. doi: 10.1016/0005-2728(77)90044-5. [DOI] [PubMed] [Google Scholar]
- McTavish H, Picorel R, Seibert M. Stabilization of isolated photosystem II reaction center complex in the dark and in the light using polyethylene glycol and an oxygen-scrubbing system. Plant Physiology. 1989;89:452–456. doi: 10.1104/pp.89.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloslavina Y, Grouneva I, Lambrev PH, Lepetit B, Goss R, Wilhelm C, Holzwarth AR. Ultrafast fluorescence study on the location and mechanism of non-photochemical quenching in diatoms. Biochimica et Biophysica Acta, Bioenergetics. 2009;1787:1189–1197. doi: 10.1016/j.bbabio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Nixon PJ. Chlororespiration. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:1541–1547. doi: 10.1098/rstb.2000.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens TG. Light-harvesting function in the diatom Phaeodactylum tricornutum. II. Distribution of excitation energy between the photosystems. Plant Physiology. 1986;80:739–746. doi: 10.1104/pp.80.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou G, Tsimilli-Michael M, Stamatakis K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynthesis Research. 2007;94:275–290. doi: 10.1007/s11120-007-9193-x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou GC, Govindjee . Advances in photosynthesis and respiration. Vol. 19. Dordrecht, The Netherlands: Springer; 2004. Chlorophyll a fluorescence: a signature of photosynthesis. [Google Scholar]
- Rappaport F, Finazzi G, Pierre Y, Bennoun P. A new electrochemical gradient generator in thylakoid membranes of green algae. Biochemistry. 1999;38:2040–2047. doi: 10.1021/bi982351k. [DOI] [PubMed] [Google Scholar]
- Rebeille F, Gans P. Interaction between chloroplasts and mitochondria in microalgae: role of glycolysis. Plant Physiology. 1988;88:973–975. doi: 10.1104/pp.88.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban A, Lavaud J, Rousseau B, Guglielmi G, Horton P, Etienne A. The super-excess energy dissipation in diatom algae: comparative analysis with higher plants. Photosynthesis Research. 2004;82:165–175. doi: 10.1007/s11120-004-1456-1. [DOI] [PubMed] [Google Scholar]
- Sandmann G, Reck H, Kessler E, Böger P. Distribution of plastocyanin and soluble plastidic cytochrome c in various classes of algae. Archives of Microbiology. 1983;134:23–27. [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ. The plastid ndh genes code for an NADH-specific dehydrogenase: isolation of a complex I analogue from pea–thylakoid–membranes. Proceedings of the National Academy of Sciences, USA. 1998;95:1319–1324. doi: 10.1073/pnas.95.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schansker G, Srivastava A, Govindjee Strasser RJ. Characterization of the 820 nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Functional Plant Biology. 2003;30:785–796. doi: 10.1071/FP03032. [DOI] [PubMed] [Google Scholar]
- Schansker G, Tóth SZ, Strasser RJ. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochimica et Biophysica Acta. 2005;1706:250–261. doi: 10.1016/j.bbabio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology. 2007;58:199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, et al. The complete nucleotide sequence of the tobacco chloroplast genome. Plant Molecular Biology Reporter. 1986;4:111–148. [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Ecophysiology of photosynthesis. Berlin: Springer-Verlag; 1994. Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis; pp. 49–70. [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochemistry and Photobiology. 1995;61:32–42. [Google Scholar]
- Ting CS, Owens TG. Photochemical and nonphotochemical fluorescence quenching processes in the diatom. Phaeodactylum tricornutum. Plant Physiology. 1993;101:1323–1330. doi: 10.1104/pp.101.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CS, Owens TG. The effects of excess irradiance on photosynthesis in the marine diatom. Phaeodactylum tricornutum. Plant Physiology. 1994;106:763–770. doi: 10.1104/pp.106.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Strasser RJ. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynthesis Research. 2007;93:193–203. doi: 10.1007/s11120-007-9179-8. [DOI] [PubMed] [Google Scholar]
- Wagner H, Jakob T, Wilhelm C. Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytologist. 2006;169:95–108. doi: 10.1111/j.1469-8137.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Volkmar P, Lohmann C, Becker A, Meyer M. The HPLC-aided pigment analysis of phytoplankton cells as a powerful tool in water quality control. Aqua (Lond.) 1995;44:132–141. [Google Scholar]
- Wilhelm C, Büchel C, Fisahn J, et al. The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist. 2006;157:91–124. doi: 10.1016/j.protis.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Duval J. Fluorescence induction kinetics as a tool to detect chlororespiratory activity in the prasinophycean alga. Mantoniella squamata. Biochimica et Biophysica Acta. 1990;1016:197–202. [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S. The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. The Plant Cell. 1999;11:43–56. doi: 10.1105/tpc.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Terashima I, Noguchi K. Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant and Cell Physiology. 2006;47:22–31. doi: 10.1093/pcp/pci219. [DOI] [PubMed] [Google Scholar]