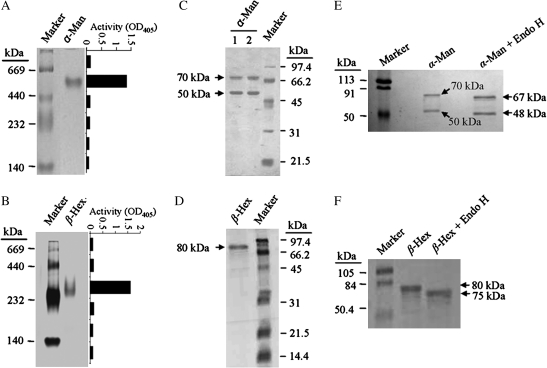
Fig. 2.
Purification and characterization of α-Man and β-Hex from capsicum fruit pericarp. (A) Purified α-Man resolved on 6% non-denaturing PAGE showing single protein band of ∼500 kDa. The graph represents the activity of α-Man determined in the corresponding gel area. (B) Purified β-Hex resolved on 6% non-denaturing PAGE showing a single protein of ∼300 kDa. The graph depicts the enzyme activity measured in the corresponding gel area. (C) Purified α-Man resolved on 12.5% SDS-PAGE showing two subunits (70 kDa and 50 kDa). Lane 1, α-Man treated with β-mercaptoethanol, lane 2, α-Man without β-mercaptoethanol treatment. (D) Purified β-Hex denatured and resolved on 12.5% SDS-PAGE, indicating a single polypeptide of 80 kDa. (E) Deglycosylation of the purified α-Man by EndoH revealed a shift as compared to the undigested. (F) EndoH digestion of the purified β-Hex confirmed the presence of glycan moiety.