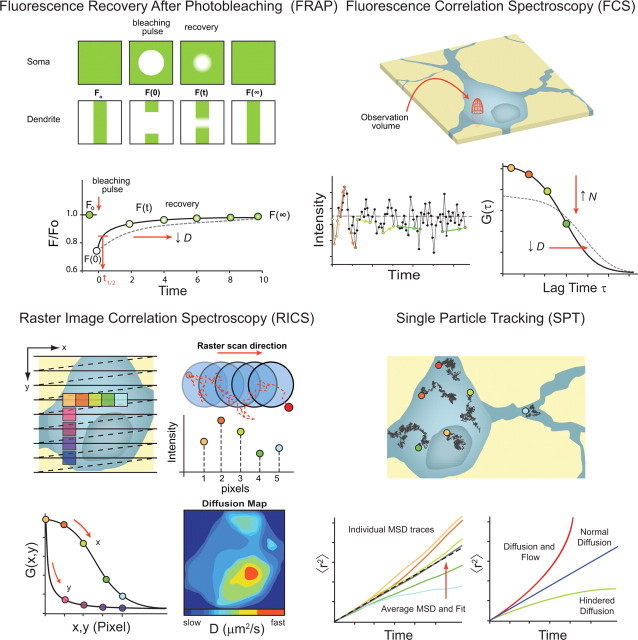
Figure 2.
Comparison of optical methods used for quantifying translational mobility in neurons. Top left, FRAP. Typically, a higher concentration of labeled probe is required for FRAP, since the technique depends on the irreversible photobleaching of a population of fluorophores and assessment of the rate of recovery back into the volume. In a FRAP experiment, a short, intense pulse of light is used to photobleach a portion of the fluorescently labeled molecules in the focal volume positioned in a region of interest, immediately after which the laser is attenuated and the rate at which new fluorophores diffuse back into the focal volume is measured. Using an appropriate model, the recovery curve is fit, and a diffusion coefficient is determined. Examples of bleaching in the soma or in a dendrite are shown (top rows). A model FRAP curve (shown below) indicates the different phases of an experiment: Fo is the baseline fluorescence intensity immediately before photobleaching, F(0) is the fluorescence intensity after photobleaching, and F(∞) is the fluorescence at the asymptote of the recovery (the color of the dots indicates the level of fluorescence being recovered). Data fitting extracts quantitative values from the recovery time course. Slower recovery (longer t1/2) represents a slower diffusion coefficient (see dashed curve). Top right, FCS. For FCS experiments, the excitation beam can be “parked” at any location in the cell. When molecules diffuse through the observation volume, florescence is emitted and the corresponding intensity fluctuations are measured over time (bottom left). The autocorrelation gives a measure of self-similarity after a certain time delay. Since the correlation of the position of any given molecule with respect to its original position decreases as the molecule diffuses, the average values of the products of points separated by shorter time intervals (orange colors points) are larger than those for longer times (green colored points). The fluctuations are then autocorrelated to produce the decay function (bottom right) that depends on the average number of molecules in the focal volume element (N) and diffusion (D). Decreasing values of D shift the autocorrelation curve to the right, while increasing N decreases the amplitude of G(τ) (see dashed curve). Bottom left, RICS. A diagram model of a raster scan on a preselected neuron is shown (top left). Horizontal lines represent the scan to collect data, and dashed lines represent the return and shift of the scanned volume between periods of data collection. The colored boxes are discrete parts of the scan used to calculate the spatial correlation of molecules moving within the sampled regions (bottom left). Molecules are captured for a longer period of time in the x dimension because of the rapid sampling in that axis, but it is less likely to find the same molecules in the spatial correlation of the y dimension because they have diffused away during the longer sampling interval between line scans. The focal volume, illustrated as blue circles, is oversampled to capture adequate information for calculating the molecules rate of movement (top right). A randomly diffusing particle is shown along with the scanned volume element (pixel), and the histogram below shows the fluorescence intensity that would be detected in each of the pixels. Fitted with the appropriate model, the number of molecules (N) and the diffusion (D) can be extracted for particular regions of interest (ROIs). By analyzing continuous ROIs through the image, a spatial map for the diffusion molecules of interest can be obtained (bottom right). In this example, diffusion is fairly homogenous through the cytoplasm but shows more rapid diffusion in the nucleus. Bottom right, SPT, accomplished with video microscopy, uses numerical algorithms to select single bright molecules on the images to “track” their position as the video elapses. This builds a map of many single trajectory paths in the same cell. Random walk traces are then converted to MSD versus time plots to analyze their transport properties (bottom left). At short timescales, single-particle traces follow nicely a linear relationship with time, but at longer times, the statistical nature of random walks allows them to deviate from one another. Thus, the standard deviation cannot be judged from a single curve but requires an average curve [see average of these 5 traces (gray line)) and its linear fit (dashed black line)]. The slope of this curve corresponds to the diffusion coefficient, and in this case the diffusion is constant. When the diffusion coefficient or the slope of MSD versus time is not constant, other types of mobility corresponding to different transport properties can be assumed, such as diffusion with flow or hindered diffusion (bottom right).