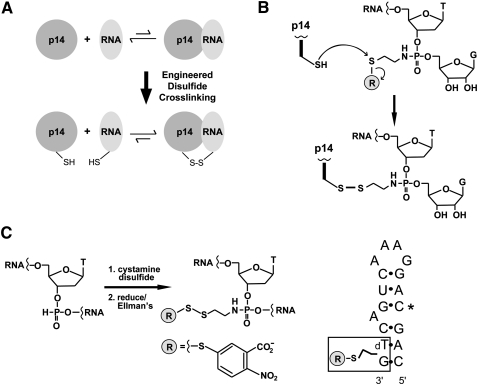
FIGURE 2.
Disulfide cross-linking strategy for interrogating the p14/SF3b155 peptide•RNA complex. (A) Cross-linking of engineered Cys containing p14/SF3b155 peptide to thiol-derivatized RNA generates a tethered RNA–protein complex in which cognate association is predicted to stabilize the disulfide bond to reduction. (B) p14•RNA cross-linking reaction. Chemistry of disulfide bond formation between single Cys containing p14/SF3b155 peptide and an N-thioalkyl modified RNA, where attachment to the backbone is mediated by single phosphoramidate subtitution for a backbone phosphate. (C) Synthesis of thiol-modified RNA. (Left) Oxidation of an H-phosphonate monomer with cystamine disulfide is followed by reduction and trapping with Ellman's reagent during the course of automated synthesis. (Right) Sequence of thiol-modifed RNA synthesized as a mimic of the pre-mRNA•U2 snRNA bulged duplex containing C in place of pseudouridine at position 4 (*).