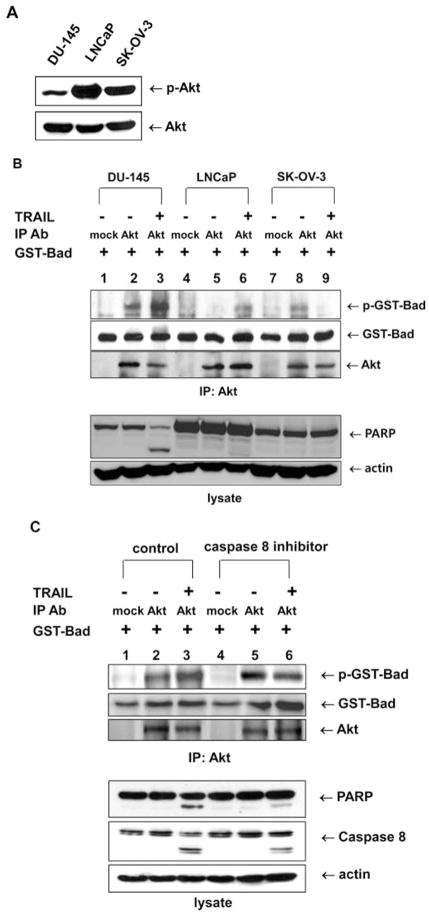
Figure 3.
Akt catalytic activity dependence on TRAIL-sensitivity and the caspase 8-dependent signaling pathway. (A) Akt phosphorylation was estimated in TRAIL-sensitive DU-145 cells and TRAIL-resistant LNCaP and SK-OV-3 cells. Each cell line was treated with 200 ng/ml TRAIL for 4 h, and lysates were immunoblotted with anti-phospho Akt antibody. (B) DU-145, LNCap and SK-OV3 cells were lysed, and the lysates were immunoprecipitated with mouse anti-Akt antibody. Akt catalytic activity was examined in vitro using GST-Bad protein as a substrate. GST-Bad and phosphorylated GST-Bad were detected with anti-Bad and anti-phospho-Ser-136-Bad antibodies, respectively. Immunoprecipitated Akt was detected with rabbit anti-Akt antibody (upper panel). Cell lysates were immunoblotted with anti-PARP or anti-actin antibody, respectively (lower panel). (C) DU-145 cells were pretreated with caspase 8 inhibitor (Z-IETD-FMK 20 μM, 30 min), followed by TRAIL treatment (200 ng/ml) for 2 h, and were lysed, and the lysates were immunoprecipitated with mouse anti-Akt antibody. Akt catalytic activity was examined in vitro using GST-Bad protein as a substrate. GST-Bad and phosphorylated GST-Bad were detected with anti-Bad and anti-phospho-Ser-136-Bad antibodies, respectively. Immunoprecipitated Akt was detected with rabbit anti-Akt antibody (upper panel). Cell lysates were immunoblotted with anti-PARP, anti-caspase 8 or anti-actin antibody, respectively (lower panel).