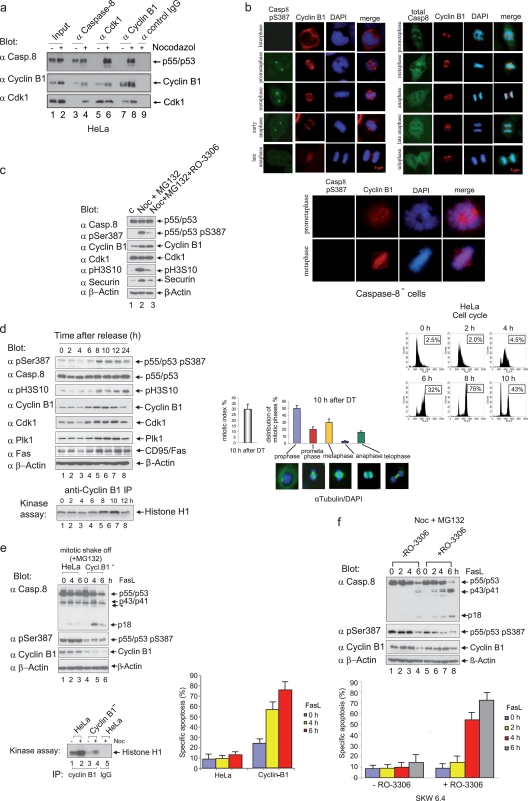
FIG. 4.
The phosphorylation of procaspase-8 correlates with Cdk1/cyclin B1 activity in vivo. (a) Lysates from asynchronous (−) or mitotic (+) HeLa cells were immunoprecipitated with caspase-8-, Cdk1-, or cyclin B1-specific antibodies. Anti-mouse IgG was used as a control. The immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted for caspase-8, cyclin B1, and Cdk1. Lanes 1 and 2 show 2.5% total input. (b) HeLa cells were arrested in G1/S using a double-thymidine block and released into fresh medium for 10 h. Semisynchronized cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) and subsequently categorized according to their staining patterns. Cells were labeled using ABcasp.-8 (pSer387), caspase-8, or cyclin B1 antibodies for analysis by microscopy. Caspase-8-deficient HeLa cells were arrested in G1/S using a double-thymidine block and released into fresh medium for 10 h. Semisynchronized cells were stained with DAPI or labeled using ABcasp.-8 (pSer387) or cyclin B1 antibodies for analysis by microscopy. (c) To enrich for mitotic HeLa cells, an incubation with nocodazole (Noc) for 16 h was carried out; cells were captured by shake-off and then treated with MG132 for 2 h (lane 2). Shake-off cells were treated as described for lane 2, except that, 1 h after MG132 addition, the Cdk1 inhibitor RO-3306 was added (lane 3). The cell lysates were immunoblotted for caspase-8, caspase-8 (pS387), cyclin B1, Cdk1, pH3S10, securin, and β-actin. (d) HeLa cells were arrested in G1/S using a double-thymidine block (time zero) and released for the times indicated. The cellular lysates were immunoblotted for caspase-8 (pS387), caspase-8, pH3S10, cyclin B1, Cdk1, Plk1, CD95/Fas, and β-actin (upper left panels). To measure the Cdk1/cyclin B1 activity in cell lysates of synchronized cells, immunoprecipitation assays were performed with cyclin B1-specific antibodies, and immunoprecipitated proteins were then subjected to kinase assays with histone H1 as the substrate (lower left panel). The cell cycle status was monitored using flow cytometry (upper right panel). Boxed numbers exhibit the percentage of cells with 4 N DNA content. The mitotic index and distribution of mitotic phases of HeLa cells 10 h after release from the G1/S block are shown: the leftmost bar graph indicates 30% mitotic index 10 h after G1/S (of these, 46% were in prophase and 25% were in metaphase). The mitotic index was determined by counting 6 times 250 to 300 cells (lower right panel). All experiments were performed in triplicate. The error bars represent the SD. (e) Characterization of mock-transfected HeLa cells and of a cyclin B1-depleted HeLa clone using a stably integrated RNAi cassette (Cycl.B1−-HeLa). Mitotic shake-off cells (mock-transfected and Cycl.B1−) were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the caspase-8 MAb C15 and antibodies for caspase-8 (pS387), cyclin B1, and β-actin (*, unspecific band). To measure Cdk1/cyclin B1 activity, immunoprecipitation assays were performed using cyclin B1-specific antibodies, and the immunoprecipitated proteins were subjected to kinase assays with histone H1 as the substrate (middle panel). Apoptosis analyses were performed by using an annexin V kit (lower panel). All experiments were performed in triplicate. Error bars represent the SD. (f) To enrich for mitotic SKW 6.4 cells, an incubation with nocodazole (Noc) for 16 h was carried out; cells were captured by shake-off and then treated with MG132 for 2 h. For the inhibition of Cdk1 activity, RO-3306 was added 1 h after MG132 treatment. Cells were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the anti-caspase-8 MAb C15. Apoptosis analyses were performed by using an annexin V kit (lower panel). All experiments were performed in triplicate. Error bars represent the SD.