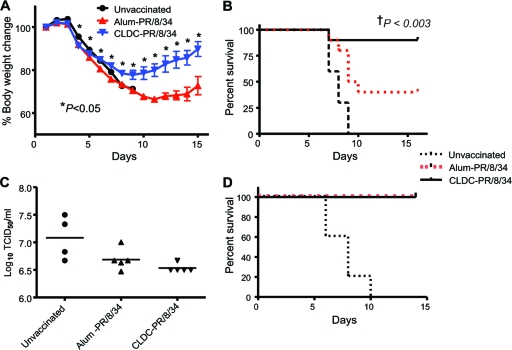
FIG. 5.
CLDC-adjuvanted influenza virus vaccine confers improved protection against a lethal heterotypic intranasal influenza virus challenge. C57BL/6J mice were vaccinated on days 1 and 7 with vaccine for influenza A virus strain PR/8/34 with CLDC as the adjuvant (CLDC-PR/8/34; n = 10) or with the same vaccine with alum as the adjuvant (Alum-PR/8/34; n = 10) or were not immunized (Unvaccinated; n = 10). Four times the LD50 of influenza A virus strain HKx31 or PR/8/34 was administered i.n. on day 10 following the second vaccination. Values for CLDC-PR/8/34-vaccinated mice were significantly different (P < 0.05) from the values for alum-PR/8/34-vaccinated mice by the two-tailed, unpaired Student t test as indicated by the asterisks. (A) Percent body weight change assessed daily from basal levels for 15 days after challenge. (B) Kaplan-Meier survival curve after a lethal HKx31 (heterosubtypic) viral challenge. †, P value comparing CLDC-PR/8/34 and Alum-PR/8/34 post-HKx31 challenge calculated using a log-rank (Mantel-Cox) test. (C) Lung influenza viral load on day 5 after a lethal HKx31 challenge. (D) Kaplan-Meier survival curve after a lethal PR/8/34 (homotypic) challenge. The results shown are representative of two independent experiments.