FIG. 7.
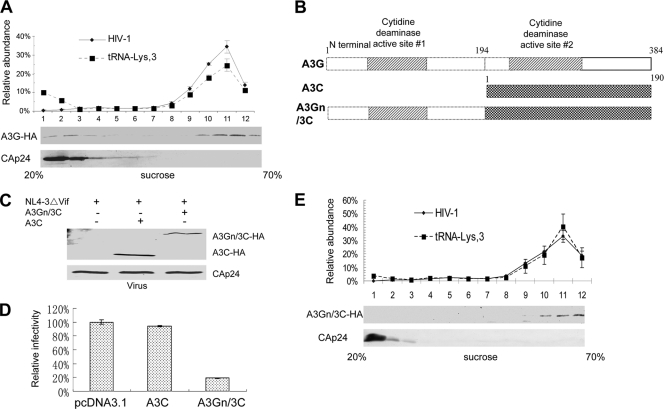
Addition of a 7SL RNA-binding domain from A3G enhances the antiviral activity of A3C. (A) A3G is associated with the viral RNP. 293T cells were transfected with NL4-3ΔVif and the A3G-HA expression vector. Viral lysates were separated, and RNA was extracted as in Fig. 5C. (B) Construction of an A3Gn/3C chimera. A3G-N and A3C were amplified, and the PCR products of A3G-N and A3C were annealed and fused using the overlapping method. (C) The A3Gn/3C chimera associates with the viral RNP as analyzed in Fig. 5C. (D) Chimeric A3Gn/3C is packaged into HIV-1 virions. The error bars indicate standard deviations. (E). A3Gn/3C exhibits increased antiviral activity against HIV-1 compared to parental A3C. Virus infectivity in the absence of A3C or A3Gn/3C was set to 100%. A3C molecules are largely associated with the viral cores but not viral RNPs. A3C cannot be targeted efficiently to the viral genomic-RNA- and primer  -containing complexes and only inefficiently induces the deamination of newly synthesized viral DNA. (3) The potent anti-HIV-1 cytidine deaminases A3G and A3F interact with 7SL RNA and are packaged efficiently into HIV-1 virions. Their association with 7SL RNA could promote their antiviral cytidine deaminase activity by strong association with viral reverse transcription complexes for efficient DNA substrate recognition and deamination.
-containing complexes and only inefficiently induces the deamination of newly synthesized viral DNA. (3) The potent anti-HIV-1 cytidine deaminases A3G and A3F interact with 7SL RNA and are packaged efficiently into HIV-1 virions. Their association with 7SL RNA could promote their antiviral cytidine deaminase activity by strong association with viral reverse transcription complexes for efficient DNA substrate recognition and deamination.