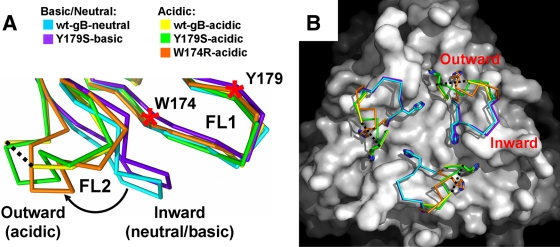
FIG. 2.
Low-pH-dependent conformational change in fusion loop FL2. (A) Overlay of the fusion loop regions, in side view. For clarity, only single protomers are shown. The inward and the outward conformations of the FL2 are labeled. The color scheme is the same as in Fig. 1C. The curved arrow indicates low-pH-induced movement of FL2. W174 and Y179 on FL1 are indicated by red asterisks. Residues 261 to 262 are missing in the wt-gB-acidic structure, and the flanking residues 260 and 263 are connected with a black dotted line. (B) Surface view of the fusion loop region. The wt-gB-neutral structure, with residues 258 to 264 and the nitrogen of Y265 omitted, is shown as a white surface. FL2 in each of the five structures is shown as a Cα trace with H263 shown as sticks. All superpositions were performed on residues 185 to 250 using Coot (12) and wt-gB-neutral trimer (chain A) as a reference molecule.