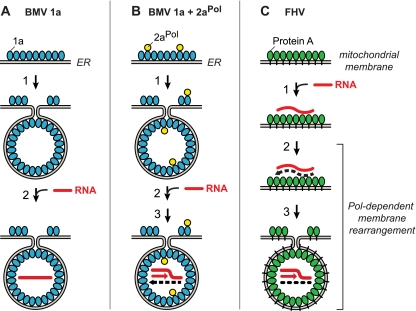
FIG. 8.
Polymerase independence and dependence of BMV and FHV spherule membrane rearrangements and replication complex assembly. (A) BMV spherule generation by multifunctional BMV RNA replication factor 1a (blue). In the absence of BMV RNA-dependent RNA polymerase 2aPol, 1a localizes to ER membranes, self-interacts, and induces the formation of ∼70-nm invaginations or spherules (39, 40, 51). In a separable subsequent reaction dependent on the activity of the C-proximal 1a NTPase/helicase domain, 1a transfers genomic RNA (red line) to the spherule interior (61). (B) In the presence of 1a and 2aPol (yellow), 1a also recruits 2aPol to ER membranes and directs spherule formation and genomic RNA recruitment as in panel A. 2aPol then synthesizes negative-strand RNA (dashed black line) that is retained in the spherule and repeatedly used as a template to synthesize new positive strands. (C) Protein A (green), the sole FHV-encoded RNA replication factor, localizes to mitochondrial outer membranes (34) and self-interacts (16) but does not induce spherule formation unless replication-competent FHV RNA templates are present and protein A's RNA polymerase domain is active (Fig. 2 to 7). Active-site mutations abolishing protein A polymerase activity or deletion of 3′ RNA replication signals still allow protein A to recognize specific 5′-proximal elements in viral RNA templates and recruit them to mitochondrial membranes (59, 60) but block RNA synthesis and invagination of FHV spherules (Fig. 7). See Discussion for additional details, including the role of FHV RNA interference suppressor B2.