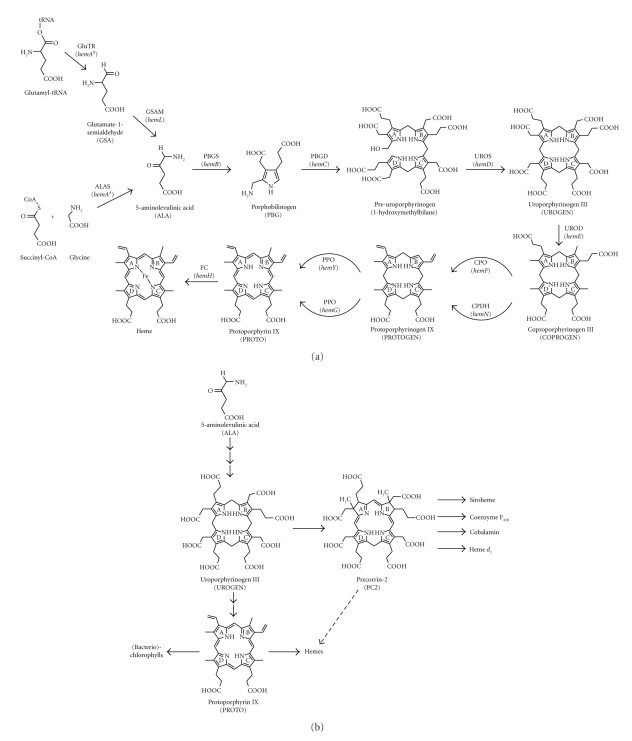
Figure 1.
Tetrapyrrole biosynthesis pathways. (a) Heme biosynthesis in most bacteria and the Eukaryota. The first common precursor in the classical heme biosynthesis pathway is ALA of which eight molecules are converted into UROGEN in three consecutive enzymatic steps. UROGEN is then further converted into heme through successive modifications of the macrocycle side chains and finally iron insertion. The enzymes involved in the classical heme biosynthesis are glutamyl-tRNA reductase (GluTR), glutamate-1-semialdehyde-2,1-aminomutase (GSAM), 5-aminolevulinic acid synthase (ALAS), porphobilinogen synthase (PBGS), porphobilinogen deaminase (PBGD), uroporphyrinogen III synthase (UROS), uroporphyrinogen III decarboxylase (UROD), oxygen-dependent coproporphyrinogen III oxidase (CPO), coproporphyrinogen III dehydrogenase (CPDH), oxygen-dependent and oxygen-independent protoporphyrinogen IX oxidase (PPO), and ferrochelatase (FC). The corresponding bacterial gene names are denoted in brackets below the enzyme names. (b) Overview of the different branches of the tetrapyrrole biosynthesis pathway. The last common precursor for the formation of all tetrapyrroles is UROGEN. Hemes and (bacterio)chlorophylls share PROTO as their last common intermediate. Siroheme, cobalamin, coenzyme F430, and heme d 1 are all biosynthesized via precorrin-2. In the Archaea and some bacteria an alternative heme biosynthesis pathway exists in which the heme is biosynthesized from precorrin-2 via as yet unknown intermediates.