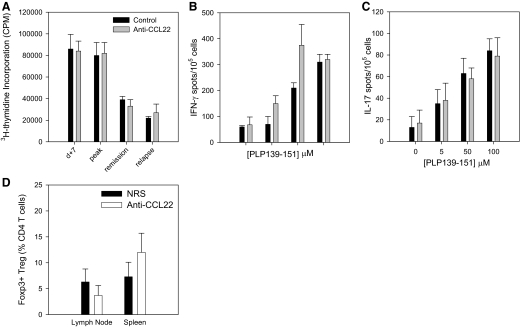
Figure 5. Anti-CCL22 does not alter peripheral antigen-specific effector T cell responses or Treg numbers.
Mice were immunized with PLP139–151 in CFA and treated with control Ig or anti-CCL22 at Days –2 and 0 relative to antigen priming. At the indicated time-points, three mice/group/time were assessed for proliferative and cytokine responses. (A) Splenic PLP139–151-specific T cell-proliferative responses were determined from control- or anti-CCL22-treated mice at Day 10 postimmunization and at times corresponding to peak clinical disease, remission, and peak-relapsing disease. The data are displayed as mean cpm (±sd), and there are no significant differences at any time-point measured. (B) Splenic PLP139–151-specific IFN-γ T cell responses were determined using ELISPOT analysis of cells from each group of mice at 10 days postimmunization. The data are displayed as mean number of cytokine-secreting cells (±sd) at each dose of peptide (0, 5, 50, and 100 nM). There are no significant differences between the two groups at any peptide dose measured. (C) Splenic, PLP139–151-specific IL-17 T cell responses were determined using ELISPOT analysis of cells from each group of mice at 10 days postimmunization. The data are displayed as mean number of cytokine-secreting cells (±sd) at each dose of peptide (0, 5, 50, and 100 μM). There are no significant differences between the two groups at any peptide dose measured. (D) The presence of Tregs in the LN and spleen was assessed by flow cytometric analysis of Foxp3 expression by CD4+CD25+-gated lymphocytes from control- or anti-CCL22-treated mice at the time when control mice showed clinical disease. The data are expressed as mean percentage of Foxp3+ cells from the CD4+ T cell population (±sd) in the LN and spleen of control (n=3)- and anti-CCL22-treated mice (n=3). There are no significant differences between the two groups in either organ analyzed. These results are representative of at least two independent experimental replicates.