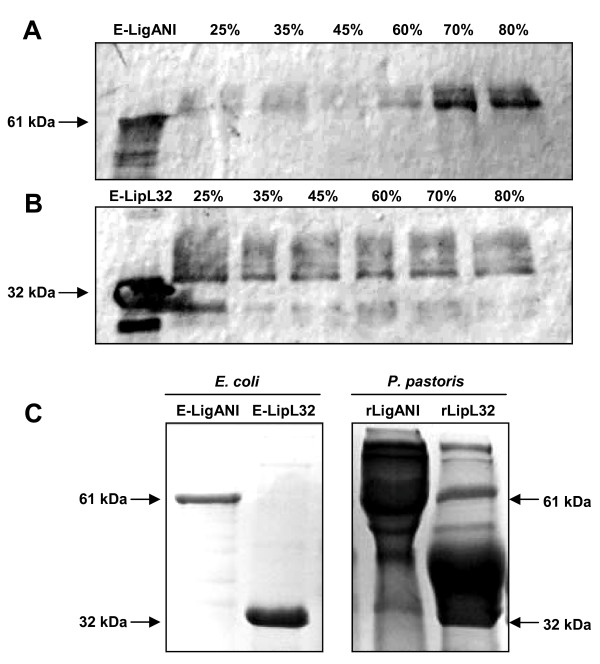
Figure 3.
Purification of rLigANI and rLipL32 expressed in P. pastoris. Recombinant proteins purified by precipitation with ammonium sulphate or by ultrafiltration. Ammonium sulphate precipitated proteins were detected by Western blotting with (A) polyclonal anti-LigANI sera or (B) an anti-LipL32 Mab. The effect of the various concentrations of ammonium sulphate (expressed as percentage values) on the precipitation of the recombinant proteins is displayed. (C) Affinity chromatography purified recombinant LigANI (61 kDa) and LipL32 (32 kDa) produced in E. coli compared to purification by ultrafiltration of rLigANI and rLipL32 secreted by P. pastoris. An equal volume (10 μL) of both proteins was loaded on the gel.