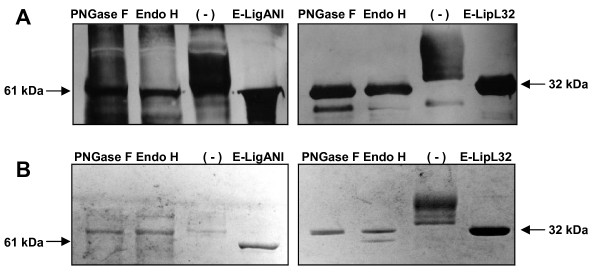
Figure 4.
Deglycosylation of rLigANI and rLipL32 produced by P pastoris. To evaluate the post-translational modification of the rLigANI and rLipL32 proteins produced and secreted by P. pastoris, the proteins were deglycosylated with PNGase F and Endo H. The resultant proteins were visualized by (A) Western blotting with polyclonal anti-LigANI sera and an anti-LipL32 Mab or by (B) SDS-PAGE stained with Coomassie blue. The proteins were digested with PNGase F, Endo H or without enzyme (-). E-LigANI (61 kDa) and E-LipL32 (32 kDa) recombinant proteins were expressed and purified from E. coli.